
U.S. Generic Injectables Pharmaceutical Contract Manufacturing Market Size, Share & Trends Analysis Report By Molecule Type (Small Molecule, Large Molecule), By Application, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68040-125-2
- Number of Pages: 100
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry:Healthcare
Report Overview
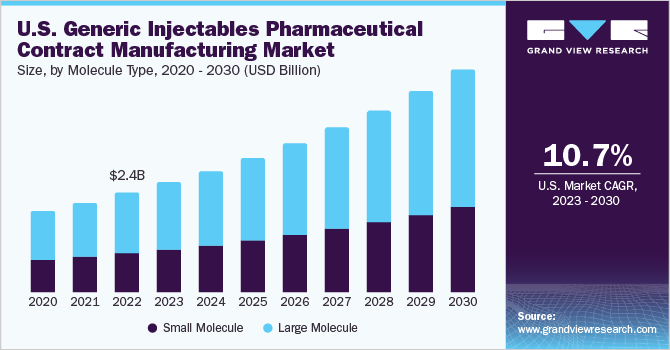
TheU.S. generic injectables pharmaceutical contract manufacturing market sizewas valued atUSD 2.39 billion in 2022and is expected to grow at a compound annual growth rate (CAGR) of 10.7% from 2023 to 2030. The rising frequency of injectable medications losing patent protection is one of the primary factors supporting the growth of the generic injectables pharmaceutical contract manufacturing space. For instance, in March 2022, Takeda Pharmaceutical Company Limited's blockbuster medication Velcade (bortezomib) saw its patent exclusivity in the U.S. expire, leading to the introduction of generic alternatives in the same year.
Furthermore, the manufacturing and supply of injectables are specialized and capital-intensive. Injectables require dedicated manufacturing lines, which can cost around USD 30-35 million. Owing to the aforementioned factors, the contract manufacturing of generic injectables is gaining traction, thus augmenting the growth of the U.S. market for generic injectables pharmaceutical contract manufacturing.
The COVID-19 pandemic significantly impacted the pharmaceutical industry, including the sector of generic injectables. It disrupted global supply chains, leading to a shortage of pharmaceutical ingredients and raw materials. This affected the manufacturing process of generic injectables, leading to potential supply shortages, excluding the injectables for COVID-19 treatment. Regulatory bodies in the U.S. prioritized the review of medical products and drugs pertaining to COVID-19 treatment, potentially delaying the approval of new generic injectables. Furthermore, economic uncertainties led to cost and pricing pressures in the pharmaceutical sector, thus affecting the pricing of generic injectables.
profoundl地缘政治战争、危机和冲突y and multifacetedly have impacted the generic injectables industry. However, the U.S. generic injectables pharmaceutical contract manufacturing sector managed to recover and achieve substantial sales growth in 2022 and 2023, largely driven by the expiration of patents for several blockbuster drugs during this period. In July 2023, the patent for Sanofi's highly successful medication Mozobil expired, leading to the introduction of generic versions of Plerixafor.
分子类型的见解乐鱼APP二维码
The large molecule segment held the dominant revenue share of 61.3% in 2022. The growing emphasis on tailored treatments and precision medicine is a major factor augmenting the growth of the large molecule generic injectable drugs segment in the U.S. Moreover, increasing product launches includingbiosimilarsis a major factor supporting segment growth. For instance, in January 2023, Amgen announced the launch of AMJEVITA, a biosimilar to Humira, in the U.S. AMJEVITA was the first generic version of Humira approved by the U.S. FDA. Such factors are poised to support segment growth.
The small molecule segment is anticipated to register a stable CAGR of 10.5% during the forecast period. Small molecule-based injectable drugs continue to hold an essential position in the treatment of a wide range of illnesses, including cancer, blood disorders, infectious diseases, and cardiovascular ailments. The high growth of the segment is majorly due to the increasing rate of generics being launched across the U.S. and, concurrently, several contract manufacturers garnering profits by entering the manufacturing business of generic injectables. This is one of the main reasons supporting segment expansion across the forecast period.
Application Insights
The oncology segment held the largest revenue share of 28.9% in 2022 in the U.S. generic injectables pharmaceutical contract manufacturing market. This is due to increasing generic product launches pertaining to cancer treatment. A considerable number of contract manufacturers are focusing on the development of generic injectables for anti-cancer treatment therapy. Additionally, a substantial pipeline of generic products for anti-cancer therapies is in development, with numerous generic drugs set to be introduced to the market.
For instance, from 2007 to 2020, the U.S. Food and Drug Administration (FDA) approved 16 oncology biosimilars. It is anticipated that this number will continue to increase in the forecast period, contributing to the expansion of this segment. Furthermore, the growing prevalence of the condition further supports research and development activities pertaining to abbreviated new drug application (ANDA) of biosimilars for anti-cancer therapeutics.
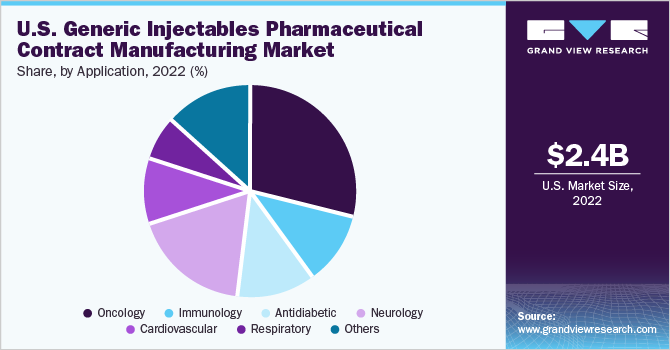
On the other hand, the neurology segment is expected to register the fastest CAGR of 11.3% during the forecast period. This is mainly due to the rise in demand for biosimilars to treat several neurological conditions. Moreover, large scale contract manufacturers are focusing on the development of neurology-based biosimilars, thereby supporting the segment’s growth.
For instance, in April 2023, Teva Pharmaceuticals and MedinCell announced that the U.S. FDA had granted approval for UZEDY (risperidone) extended-release injectable suspension for treating schizophrenia in adults. Additionally, the incidence of neurological disorders such as Alzheimer's disease and Parkinson's disease has been steadily increasing over the past decade, which is further anticipated to support the growth of this segment.
Key Companies & Market Share Insights
Major industry players focus on expanding their facilities, collaborations, partnerships, and mergers and acquisitions. These are the main strategic initiatives influencing the industry dynamics. For instance, in March 2023, PCI Pharma Services, a biopharmaceutical contract manufacturer, announced its intention to invest USD 50 million in expanding its sterile injectables facility located in Rockford, Illinois. Some prominent players in the U.S. generic injectables pharmaceutical contract manufacturing market include:
Hikma Pharmaceuticals plc
Pfizer Inc.
Fresenius Kabi
Sandoz AG
Jubilant Pharma Limited
Baxter
PCI Pharma Services
Gland Pharma Limited (USA)
Dr. Reddy’s Laboratories Ltd.
Grand River Aseptic Manufacturing
U.S. Generic Injectables Pharmaceutical Contract Manufacturing Market Report Scope
Report Attribute |
Details |
Market size value in 2023 |
USD 2.64 billion |
Revenue Forecast in 2030 |
USD 5.39 billion |
Growth rate |
CAGR of 10.7% from 2023 to 2030 |
Base year for estimation |
2022 |
Historical data |
2018 - 2021 |
Forecast period |
2023 - 2030 |
Quantitative units |
Revenue in USD Million and CAGR from 2023 to 2030 |
Report Coverage |
Revenue forecast, company share, competitive landscape, growth factors, and trends |
Segments Covered |
Molecule type, application |
Country scope |
U.S. |
Key companies profiled |
Hikma Pharmaceuticals plc; Pfizer Inc.; Fresenius Kabi; Sandoz AG; Jubilant Pharma Limited; Baxter; PCI Pharma Services; Gland Pharma Limited (USA); Dr. Reddy’s Laboratories Ltd.; Grand River Aseptic Manufacturing |
15% free customization scope (equivalent to 5 analyst working days) |
If you need specific market information, which is not currently within the scope of the report, we will provide it to you as a part of customization |
革命制度党cing and purchase options |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
U.S. Generic Injectables Pharmaceutical Contract Manufacturing Market Report Segmentation
This report forecasts revenue growth at the country level and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this study, Grand View Research has segmented the U.S. generic injectables pharmaceutical contract manufacturing market report on the basis of molecule type and application:
分子类型前景(梦nue, USD Million, 2018 - 2030)
Small Molecule
Large Molecule
Application Outlook (Revenue, USD Million, 2018 - 2030)
Oncology
Immunology
Antidiabetic
Neurology
Cardiovascular
Respiratory
Others
Frequently Asked Questions About This Report
b.The U.S. generic injectables pharmaceutical contract manufacturing market size was valued at USD 2.39 billion in 2022 and is expected to reach USD 2.64 billion in 2023.
b.The U.S. generic injectables pharmaceutical contract manufacturing market is expected to grow at a compound annual growth rate (CAGR) of 10.7% from 2023 to 2030 to reach USD 5.39 billion by 2030.
b.Based on molecule type segment, the large molecule segment accounted for the largest revenue share of 61.3% in 2022. The growing emphasis on tailored treatments and precision medicine is one of the major factors augmenting the growth of the large molecule generic injectable drugs segment in the U.S.
b.Some of the key players operating in the market include Hikma Pharmaceuticals PLC, Pfizer Inc., Fresenius Kabi, Sandoz AG, Jubilant Pharma Limited, Baxter, and a few others.
b.A few factors driving the growth of this market are the rising frequency of patent expirations for blockbuster drugs, streamlined regulatory pathways and low cost of generics, and increasing penetration of CDMOs in the generic injectables space.





