
Transcatheter Heart Valve Market Analysis By Application (Transcatheter Aortic Valve, Transcatheter Pulmonary Valve, Transcatheter Mitral Valve), By Technology (Balloon Expanded Transcatheter Valve, Self-Expanded Transcatheter Valve), And Segment Forecasts To 2024
- Report ID: GVR-1-68038-096-5
- 页数:81
- Format: Electronic (PDF)
- Historical Data: 2013-2015
- Industry:Healthcare
The market is driven by the introduction of novel products such as third-generation heart valves
The global transcatheter heart valve market size was valued at nearly USD 1.93 billion in 2015 and is expected to reach over USD 8.62 billion by 2024. The key reasons attributing to the high growth include the upsurge in the prevalence of valvular heart diseases amongst the population above 65 years of age, introduction of third-generation transcatheter heart valves, and favorable reimbursement policies in the U.S. and European Union (EU) nations.
Increasing mortality due to chronic and end-stage cardiovascular diseases in the elderly population is a key concern for healthcare providers across the world. As per the American Heart Association estimates in 2015, nearly 17.3 million deaths occur each year due to various cardiovascular diseases. In the U.S., heart disease is a leading cause of death, accounting for approximately 375,000 deaths per year.
Rising geriatric population is leading to a significant burden on the healthcare system. As per the UN estimates, the geriatric population in the U.S. is expected to reach over 72 million by 2022. The growth of the target population coupled with growing risk of end-stage cardiovascular diseases is expected to improve the usage of these valves. Moreover, the benefits of minimally invasive surgeries and transcatheter heart valve implantation have been strongly advocated on the basis of PARTNER I and PARTNER II clinical trials.
North America transcatheter heart valve market, by application, 2013 - 2024 (USD Million)
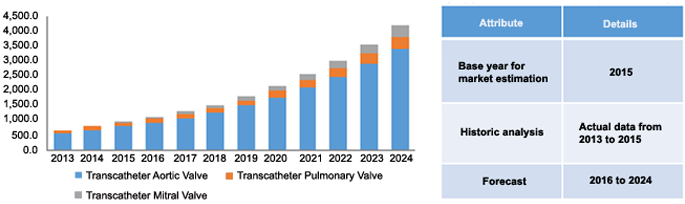
Furthermore, presence of favorable reimbursement policies available to the patients in the form of government-sponsored Medicare, Medicaid plans, and individual insurance policies, are expected to boost the treatment rates for aortic stenosis, mitral regurgitation, & tricuspid regurgitation, and these developments are expected to positively reinforce the transcatheter heart valve market expansion over the forecast period. Medicare National Coverage Determination is one such policy issued by CMS that allows the coverage of these heart valves under Coverage with Evidence Development.
The transcatheter aortic valve was the application market
Key application segments include transcatheter aortic valve, pulmonary valve, and mitral valve. In 2015, the transcatheter aortic valve segment accounted for the maximum share of 83.31%, owing to the growing healthcare expenditure, increasing prevalence of chronic heart diseases, and increasing number of people with severe aortic stenosis. Moreover, technological advancements pertaining to the development of the tricuspid valve intervention, implementation of harpoon, and neochord systems are also amongst the key factors that are expected to boost usage rates over the forecast period.
此外,不同的临床试验进行demonstrate the significance of transcatheter aortic valve replacement therapy for the treatment of inoperable patients with aortic stenosis or other valvular diseases have enabled faster adoption of this technology across the U.S. and EU nations. For instance, PARTNER II clinical trial pertaining to the Edwards Sapien 3 study involving over 1000 patients at around 50 locations in Canada and the U.S., demonstrated more desirable outcomes pertaining to various primary endpoints such as stroke, mortality, and the ability to serve moderate-to-severe valve regurgitation.
However, the transcatheter mitral valve segment is expected to have the highest CAGR of 23.0% over the forecast period. The U.S. FDA approval of the transcatheter mitral repair device in October 2013 is a key driver of the growth of this segment. Growing awareness regarding the benefits of minimally invasive heart valve surgeries and increasing demand for new treatment options for mitral regurgitation are expected to drive the usage rates over the forecast period.
The balloon expanded transcatheter valve is the fastest growing technology
In 2015, the self-expanded transcatheter valve market accounted for a maximum share of 65.6%. Most of the first and second-generation transcatheter heart valves are based on this technology and, therefore, account for the maximum market share. However, due to technological advancements, increasing awareness regarding the benefits of balloon-expandable valve systems, and improvements in clinical outcomes, the balloon catheters segment is expected to have the fastest CAGR of nearly 17% over the forecast period.
The Asia Pacific market to grow at the highest rate during the forecast period
In 2015, North America accounted for the majority share of nearly 49%. The major factors contributing to its high market share include the increasing investment in R&D and growing awareness about advanced transcatheter heart valve therapy. The U.S. accounts for the majority share in North America and the U.S. FDA approval for these products is a critical milestone for commercialization. Moreover, the robust reimbursement framework, incentives for new product development, and collaboration between various stakeholders are additional factors driving growth in this region.
然而,亚太地区预计将增长与maximum CAGR of 17.8% over the forecast period. Japan and China are few of the key hubs for the medical devices market. The growing demand for innovative heart valve therapy devices, rapidly developing the healthcare sector, increasing geriatric population, and rising healthcare expenditure are among the few factors that are expected to boost the transcatheter heart valve market growth. For instance, in some states of India, the presence of favorable government initiatives and reimbursement policies such as Aarogyasri, Jeevandayee Arogya Yojana, and Kalaignar insurance scheme provide coverage for the transcatheter heart therapies.
Edwards Lifesciences Corporation is the most dominant player in the transcatheter heart valves market
Some of the key players in the transcatheter heart valve market include Boston Scientific Corporation, Bracco Group, Braile Biomedica, Direct Flow Medical, Edwards Lifesciences, JenaValve, St. Jude Medical, Symetis, and ValveXchange, Inc. The market is oligopolistic in nature and is dominated by Edwards Lifesciences, Medtronic, and St. Jude Medical. These companies are actively scouting for potential start-ups and are also collaborating with different research centers for new product development. For instance, Edwards Lifesciences Corporation acquired CardiAQ Valve Technologies, Inc. to expand into the mitral valve replacement market.
Transcatheter Heart Valve Market Report Scope
Report Attribute |
Details |
Market size value in 2020 |
43.4亿美元 |
Revenue forecast in 2024 |
USD 8.62 billion |
Growth Rate |
CAGR of 16.0% from 2016 to 2024 |
Base year for estimation |
2015 |
Historical data |
2013 - 2015 |
Forecast period |
2016 - 2024 |
Quantitative units |
收入在美国D million and CAGR from 2016 to 2024 |
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
Segments covered |
Application, technology, region |
Regional scope |
North America; Europe, Asia Pacific; Latin America; Middle East & Africa |
Country scope |
U.S.; Canada; Germany; U.K.; China; Japan; Brazil; Mexico; South Africa |
Key companies profiled |
Boston Scientific Corporation; Bracco Group; Braile Biomedica; Direct Flow Medical; Edwards Lifesciences; JenaValve; St. Jude Medical; Symetis; ValveXchange, Inc. |
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
革命制度党cing and purchase options |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
Frequently Asked Questions About This Report
b.The global transcatheter heart valve market size was estimated at USD 3.68 billion in 2019 and is expected to reach USD 4.34 billion in 2020.
b.The global transcatheter heart valve market is expected to grow at a compound annual growth rate of 16.0% from 2017 to 2024 to reach USD 8.61 billion by 2024.
b.Transcatheter aortic valve application dominated the transcatheter heart valve market with a share of 82.9% in 2019. This is attributable to increasing healthcare expenditure, growing prevalence of chronic heart diseases, and a rise in the number of people with severe aortic stenosis.
b.Some key players operating in the transcatheter heart valve market include Boston Scientific Corporation, Bracco Group, Braile Biomedica, Direct Flow Medical, Edwards Lifesciences, JenaValve, St. Jude Medical, Symetis, and ValveXchange, Inc.
b.Key factors that are driving the market growth include increasing prevalence of valvular heart diseases, the introduction of third-generation transcatheter heart valves, and favorable reimbursement policies in the U.S. and European Union (EU) nations.





