
Sterile Injectable Contract Manufacturing Market Size, Share & Trends Analysis Report By Molecule Type, By Route Of Administration, By Therapeutic Application, By End-use, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68040-097-0
- Number of Pages: 195
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry:Healthcare
Report Overview
The globalsterile injectable contract manufacturing market sizewas estimated atUSD 12.89 billion in 2022and is projected to grow at a compound annual growth rate (CAGR) of 12.1% from 2023 to 2030. The development of biopharmaceuticals, such as vaccines and cell and gene therapies, is boosting the production of injectable drug products in the global market. Moreover, the increasing prevalence rate of chronic illnesses, along with faster approval of sterile injectables compared to other drug types, are further supporting the need for injectables.
The high demand for sterile injectables has led to the growth in outsourced agreements amongst contract manufacturers and original drug sponsors, thereby augmenting the growth of the sterile injectable contract manufacturing industry. Rapid absorption, lower risk of drug degradation by gastric secretion, faster drug action, and less concentration of the drugs are among the parameters that support the demand for commercial injectable doses.
Pharmaceutical companies engaged in injectable manufacturing are gaining traction for offering development support for large molecule injectable drugs, monoclonal antibody therapies, and infectious disease treating drugs. Factors such as shorter and more economical research and development cycles of generic injectable and the increasing advancements in treatment options for rare diseases are augmenting the growth of injectable contract manufacturers in the global market.
COVID-19爆发有积极的影响sterile injectable contract manufacturing industry. From 2020 to 2021, the demand for COVID-19 vaccines saw a significant surge because of the contagious and rapidly spreading infection. Thus, to satisfy the growing demand for COVID-19 vaccines, several contract manufacturers started to provide bulk manufacturing services for the therapeutic dosage.
Hence, the aforementioned factors led to a positive impact of the COVID-19 pandemic on the sterile injectable contract manufacturing industry. Furthermore, the market was considerably impacted by the geopolitical war between Ukraine and Russia. Russia’s invasion of Ukraine had a global effect on several industries including the pharmaceutical industry also.
Several pharmaceutical giants and other established contract manufacturers suffered various difficulties including delays throughout the drug development process, risk of non-compliance for on-market products, and loss of business continuity. Moreover, several global pharmaceutical companies have scaled down their manufacturing operations in Russia. The rising inflation globally is another significant macroeconomic factor leading to a decline in the number of drug approvals in 2022, thereby negatively affecting the pharmaceutical business.
This also implies that the decline in the new molecular entity (NME) approvals will result in a direct decrease in the commercial scale production agreements in 2023 for contract manufacturers. In 2022, the U.S. FDA approved approximately half of the most innovative drugs than that compared to 2021. The drop in these NMEs, both biologics and small molecules, was majorly due to rising inflation globally, which has been further triggered by the outbreak of the geopolitical war simultaneously.
Molecule Type Insights
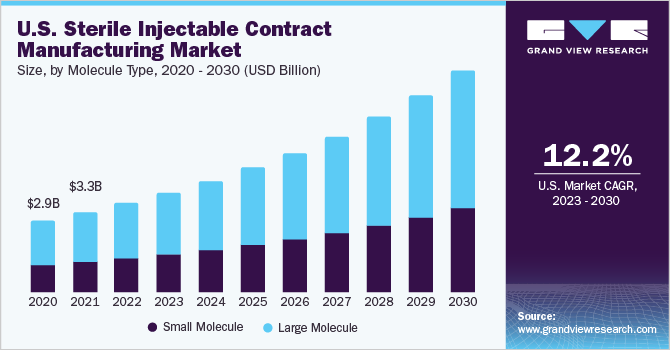
The small molecule segment is anticipated to witness a stable CAGR of 11.9% from 2023 to 2030. The segment is driven by the globally increasing demand for generic-based injectables, which includes small molecules. The rising pipeline of small molecules is a prominent factor supporting the stable growth of the segment during the forecast period. However, the injectables such as vaccines majorly constitute large molecules. Thus, the small molecules segment held a limited market share in 2022. However, the increasing adoption of advanced technology has led to the growth of small molecule-based injectables in recent years, thereby augmenting the segment’s growth.
The large molecule segment dominated the sterile injectable contract manufacturing industry and accounted for the largest revenue share of 61.3% in 2022. The high growth of this segment is attributed to the rising investments by contract manufacturers in the development of large molecule-based therapeutics, the increasing pipeline of injectables in biologics, and a surge in the U.S. FDA approvals forbiosimilars. Furthermore, the segment is anticipated to witness lucrative growth during the analysis period owing to an increasing number of biologics being in the pipeline, which is estimated to be launched between 2024 to 2027.
For instance, as per the U.S. FDA,biologicscurrently consume approximately 44% to 45% of the development pipeline from 2023 to 2024. Hence, the significant growth across the pipeline of large molecules categories is anticipated to augment the growth of the market for sterile injectable contract manufacturing. In addition, the rapid rise in the development pipeline of cell & gene therapies is among the most crucial factors supporting the demand for large molecule-based injectables across the globe.
For instance, mentioned below is the global status of the gene therapy pipeline as of June 2022:
Global Status as of June 2022 |
Number of Therapies |
|
Q4 2021 |
Q4 2022 |
|
Preclinical |
1,412 |
1,515 |
Phase I |
248 |
254 |
Phase II |
244 |
248 |
Phase III |
32 |
30 |
Pre-registration |
5 |
6 |
Total number of therapies (preclinical to pre-registration) |
1,941 |
2,053 |
Source: Industry Journals, Investor Presentations, Primary Interviews, Grand View Research, American Society for Gene + Cell Therapy
Therapeutic Application Insights
The cancer segment dominated the market for sterile injectable contract manufacturing and accounted for the largest revenue share of 28.5% in 2022. The dominance of the segment is majorly due to the increasing focus on the development of anti-cancer therapeutics. The increasing pipeline of anti-cancer drugs, growing research & development activities for treating critical cancer types, and growing approval of oncology drugs are among the few considerable factors supporting the segment’s robust revenue shares in the sterile injectable contract manufacturing industry. For instance, in July 2022, Dr. Reddy’s laboratories announced the launch of Bortezomib for injection, used in treating multiple myeloma and mantle cell lymphoma.
The central nervous system diseases segment, on the other hand, is anticipated to witness a lucrative CAGR of 12.9% during the analysis period. The high growth of the segment is majorly due to the increasing number of clinical research in diseases associated with the central nervous system. For instance, in January 2023, Eisai Co., Ltd. and Biogen Inc. announced that the U.S. FDA has approved lecanemab-irmb 100 mg/mL injection for intravenous use for the treatment of Alzheimer's disease (AD). Moreover, an increasing number of biopharmaceutical companies penetrating the contract manufacturing industry is another significant factor supporting the growth of the segment.
Route Of Administration Insight
静脉注射(IV)类为主的杂志e injectable contract manufacturing industry with the largest revenue share of 32.2% in 2022. This is attributable to the large number of sterile injectables approved for administration through intravenous routes. The segment is also poised to witness stable growth across the forecast period owing to its robust pipeline of IV dosage, which is anticipated to be launched shortly. The FDA announced that the total volume of injectables launched in 2021 majorly constituted IV infusion dosages, which was approximately 30-35% of the total injectable volume. Hence, the aforementioned factors are strongly supporting the dominance of the segment.
On the other hand, the subcutaneous (SC) segment is anticipated to witness the fastest CAGR of 12.6% during the forecast timeframe. High growth is primarily due to its high demand, which is attributable to its ability to administer numerous types of medications for various medical conditions. As subcutaneous tissue has few blood vessels, the injected drug is diffused slowly at a sustained absorption rate. Therefore, it is highly effective in administeringvaccines, growth hormones, and insulin, which require continuous delivery at a low dose rate. Hence, the associated benefits of the SC route of administration are the major factors promoting its demand, thereby augmenting the segment’s growth.
End-use Insight
Biopharmaceutical companies comprised the largest revenue share of 45.9% in 2022. This segment is expected to dominate the sterile injectable contract manufacturing industry during the analysis period owing to the increasing trend of end-to-end outsourcing services, especially among small- and mid-size pharmaceutical companies lacking expertise in bulk capacity manufacturing. Furthermore, the growing number of biologics in the pipeline, coupled with the rising trend of outsourcing in biopharmaceutical companies is expected to drive growth of this segment over the forecast period.
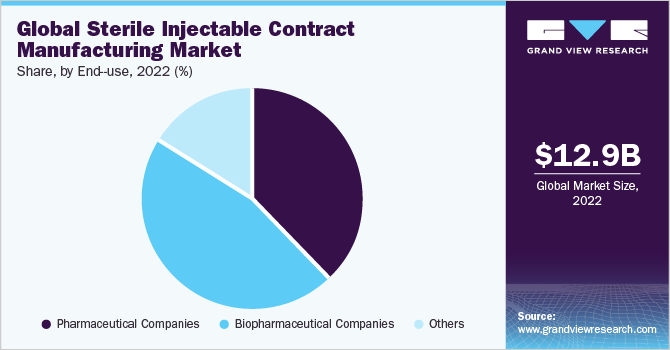
On the other hand, pharmaceutical companies are anticipated to witness a stable CAGR of 12.2% from 2023 to 2030. The segment's high growth is majorly due to the growing number of pharmaceutical companies penetrating the vaccine industry, thereby augmenting its share. Moreover, increasing investments across drug development by major pharmaceutical giants to manufacture novel rare disease therapeutics is another significant factor supporting the segment’s growth.
Regional Insight
North America accounts for the largest share of 36.1% in 2022, owing to the presence of established CMOs specializing in injectable manufacturing services, such as Baxter Biopharma and Catalent Inc. The U.S. is the biggest market for injectable outsourcing as several biopharmaceutical companies prefer outsourcing their manufacturing services to CMOs based in the U.S. due to their delivery of quality products/services. Moreover, stringent regulatory policies regarding the inspection of manufacturing facilities are anticipated to promote domestic contract manufacturing. In addition, growing R&D investments by life sciences and pharmaceutical companies are anticipated to increase the demand for sterile injectable contract manufacturing in the region.
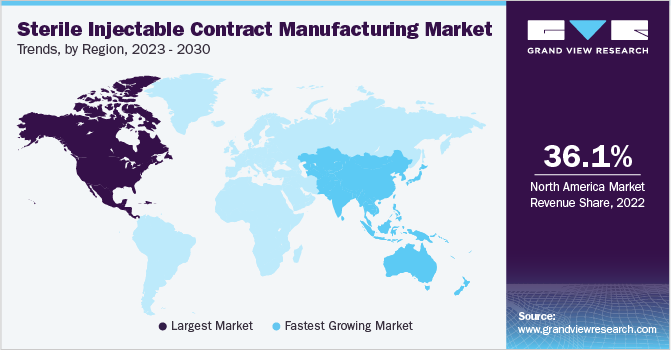
Asia Pacific is anticipated to register the fastest CAGR of 13.1% during the forecast period. Asia Pacific is one of the most attractive markets for contract manufacturing. This is due to cost-saving opportunities in Asian countries and supportive regulatory reforms, especially in India and China. In 2016, as part of the Marketing Authorization Holder (MAH) system reforms, China’s FDA legalized contract manufacturing, thus allowing domestic biopharmaceutical & pharmaceutical companies access to contract manufacturers, significantly supporting the region’s market growth.
In addition, the presence of EMA- and FDA-approved facilities in the country is also anticipated to increase foreign investments. For instance, approximately 92 contract service organizations have headquarters in Jiangsu, with 72% of the facilities having EMA and FDA approval. Thus, the high number of EMA- and FDA-approved facilities is anticipated to boost the market growth of sterile injectable contract manufacturing.
Key Companies & Market Share Insights
The major players operating across the market for sterile injectable contract manufacturing are focused on adopting in-organic strategic initiatives such as mergers, partnerships, acquisitions, etc. Moreover, companies are focusing on expanding their production capacity to position themselves in the market better. For instance, in March 2023, PCI Pharma Services announced an investment of USD 50 million for a new 200,000ft manufacturing facility in Illinois, US. The site is expected to enhance the contract development capacity in injectables of biologics and small molecules. Some prominent players in the global sterile injectable contract manufacturing market are:
Baxter
Catalent, Inc.
Vetter Pharma
Recipharm AB
Aenova Group
Fresenius Kabi
Unither Pharmaceuticals
Famar
Cipla Inc.
NextPharma Technologies
Sterile Injectable Contract Manufacturing Market Report Scope
Report Attribute |
Details |
Market size value in 2023 |
USD 14.35 billion |
Revenue forecast in 2030 |
USD 31.92 billion |
Growth rate |
CAGR of 12.1% from 2023 to 2030 |
Base year for estimation |
2022 |
Historical data |
2018 - 2021 |
Forecast period |
2023 - 2030 |
Quantitative units |
Revenue in USD million/billion and CAGR from 2023 to 2030 |
Report coverage |
Revenue forecast, company share, competitive landscape, growth factors, and trends |
Segments covered |
Molecule type, therapeutic application, route of administration, end-use, region |
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
Country scope |
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; India; Japan; Australia; Thailand; South Korea; Brazil; Mexico; Argentina; South Africa, Saudi Arabia; UAE; Kuwait |
Key companies profiled |
Baxter; Catalent, Inc.; Vetter Pharma; Recipharm AB; Aenova Group; Fresenius Kabi; Unither Pharmaceuticals; Famar; Cipla Inc.; NextPharma Technologies |
Customization scope |
If you need specific market information, which is not currently within the scope of the report, we will provide it to you as a part of customization |
价格和购买该俱乐部ns |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
Global Sterile Injectable Contract Manufacturing Market Report Segmentation
This report forecasts revenue growth at global, regional & country levels and provides an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global sterile injectable contract manufacturing market report based on molecule type, therapeutic application, route of administration, end-use, and region:
Molecule Type Outlook (Revenue, USD Million, 2018 - 2030)
Small Molecule
Large Molecule
Therapeutic ApplicationOutlook (Revenue, USD Million, 2018 - 2030)
Cancer
Diabetes
Cardiovascular Diseases
Central Nervous System Diseases
Infectious Disorders
Musculoskeletal
Anti-viral
Others
Route of AdministrationOutlook (Revenue, USD Million, 2018 - 2030)
Subcutaneous (SC)
Intravenous (IV)
Intramuscular (IM)
Others
End-use Outlook (Revenue, USD Million, 2018 - 2030)
Pharmaceutical Companies
Biopharmaceutical Companies
Others
Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
U.S.
Canada
Europe
UK
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
Australia
South Korea
Thailand
Latin America
Brazil
Mexico
Argentina
Middle East & Africa
South Africa
Saudi Arabia
UAE
Kuwait
Frequently Asked Questions About This Report
b.The global sterile injectable contract manufacturing market size was estimated at USD 12.89 billion in 2022 and is expected to reach USD 14.35 billion in 2023.
b.The global sterile injectable contract manufacturing market is expected to grow at a compound annual growth rate of 12.1% from 2023 to 2030 to reach USD 31.92 billion by 2030.
b.North America dominated the sterile injectable contract manufacturing market with a share of 36.1% in 2022. This is attributable to the presence of established CMOs specializing in injectable manufacturing services, such as Baxter Biopharma and Catalent Inc.
b.Some key players operating in the sterile injectable contract manufacturing market include Baxter, Catalent, Inc., Vetter Pharma, Recipharm AB, Aenova Group, Fresenius Kabi. etc.
b.Increasing pipeline and approvals of injectables and growing demand for biologics and biosimilars significantly supported the market growth.





