
Nordic Regulatory Affairs Market Size, Share & Trends Analysis Report By Service, By Service Provider, By Company Size, By Category, By Product Stage, By Indication, By End-use, By Country, And Segment Forecasts, 2021 - 2028
- Report ID: GVR-4-68039-331-1
- Number of Pages: 114
- Format: Electronic (PDF)
- Historical Range: 2016 - 2019
- Industry:Healthcare
Report Overview
The Nordic regulatory affairs market size was valued at USD 153.2 million in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 7.5% from 2021 to 2028. Changing regulatory landscape, increasing demand for the faster approval process, and economic and competitive pressures are some of the key factors expected to drive the market. The entry of life sciences companies in the Nordic markets, especially in countries such as Denmark and Sweden, for R&D collaborations and the evolution of new areas, such as orphan drugs, biosimilars, advanced therapy medicinal products (ATMPs), and personalized medicine, are further anticipated to contribute to the market growth. This is because new areas would require advanced technical expertise for compliance with regulatory requirements.
For instance, the development and use of biosimilars continue to grow in the Nordic region. Countries, especially Sweden and Norway, are known to hold leadership positions in crafting policies that facilitate the production of biosimilars. Thus, for faster approval of their biosimilar drugs, companies have to rely on clearance from regulatory bodies, thus creating demand for services in the Nordic countries.
At present, the healthcare industry is focused not only on the development of blockbuster therapies for the treatment of various diseases but also on targeted gene therapies, especially drugs and precision medicine, which help to treat specific diseases and disorders. Some of these products are also being combined with medical devices to enhance the quality of drug delivery and patient monitoring or adherence, thus increasing the complexity of defining the regulatory strategy and pathway to market.
针对这一点,在2018年,瑞典基因组医学(GMS) was launched for the development of precision medicine in Sweden. It aimed at implementing precision medicine into the clinical trial settings in the country. In June 2020, Vinnova, an innovation agency, invested USD 4.4 million to promote R&D and collaborations in the field of precision medicine. Precision medicine is a potential area of growth in Sweden. Stockholm, the country’s capital, is home to over 50% of companies focused on the area of precision medicine. Many of these companies are actively pursuing international collaboration and partnerships. Thus, compliance withregulatory affairsduringclinical trialsand product approvals is expected to boost demand for regulatory affairs in the Nordic countries.
The sudden spread of the COVID-19 pandemic affected the ongoing clinical trials at the global level. The impact was also visible in the Nordic countries, such as Norway, Sweden, and Denmark. In Norway, a temporary halt of patient recruitment for new clinical trials was seen. Adherence to new mandates released by each country’s government agency has further created challenges to continue ongoing clinical trials.
Furthermore, with new guidelines being imposed, several companies are taking assistance of CROs, thus increasing demand for regulatory affairs in the Nordic countries. In addition, R&D for the development of vaccines and drugs is ongoing in these Nordic countries. For instance, since the outbreak of COVID-19, 260 clinical studies relating to COVID-19 have been approved by the Swedish Ethical Review Authority. Thus, the need for regulatory affairs is expected to increase in the Nordic countries.
Category Insights
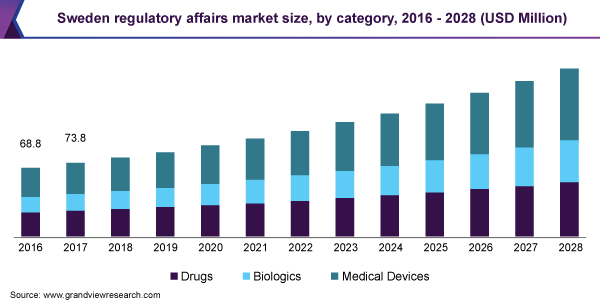
The medical devices segment accounted for the largest revenue share of 39.2% in 2020. This can be attributed to the increased competition due to the high demand for wearables and technological advancements in material science, design languages, personalized healthcare, and additive manufacturing. This eventually encourages manufacturers and technology developers to focus on core competencies and outsource non-core activities. Based on the category, the market is segmented into drugs, biologics, and medical devices. The medical devices category is sub-segmented into diagnostics and therapeutics.
进一步划分为英诺华药物段tor and generic while the biologics segment is sub-segmented into biosimilars, ATMPs, and biotech products. Innovator drugs dominated the market in 2020. The biologics segment is anticipated to register the fastest growth rate of 8.6% over the forecast period. The growing number of product approvals, increasing demand for targeted therapies, and the rise in the development of biosimilars are anticipated to contribute to segment growth.
Indication Insights
The oncology segment dominated the market in 2020 and accounted for a revenue share of 33.1%. The segment will expand further on account of the increasing prevalence of chronic diseases, such as cancer, along with the presence of several innovative pipeline products. In Sweden, oncology holds the leading position in terms of drug development activity.
The immunology segment is anticipated to register the fastest CAGR of 9.0% over the forecast period. The growing use of immunological molecules in cancer therapy, coupled with their potential in facilitating the treatment of various cardiovascular, neurological, and inflammatory diseases, is anticipated to boost segment growth.
Product Stage Insights
The clinical studies segment led the market and accounted for a 46.2% share in 2020. The increasing prevalence of chronic diseases and the emergence of new diseases are anticipated to increase the number of clinical trials. Furthermore, several companies conduct clinical trials on existing molecules for either indication expansion or combination therapy. For instance, many companies are running clinical trials for the use of previously approved anti-viral, antibiotics, corticosteroids, and monoclonal antibodies to treat Covid-19. In November 2020, Eli Lilly's monoclonal antibody received the FDA approval for COVID-19. Such activities are anticipated to increase the need for regulatory services related to medical writing and regulatory labeling.
The preclinical segment is anticipated to expand at the fastest CAGR during the forecast period. This can be attributed to the increasing number of molecules in the preclinical stage, especially in recent times, as companies are lined up for the development and approval of vaccines and drugs to treat COVID-19.
Service Provider Insights
The outsourcing segment accounted for the largest revenue share of 59.9% in 2020 and is anticipated to expand at the fastest CAGR during the forecast period. Several medical devices, pharmaceutical, and biotechnology startups generally lack the budget and infrastructure for a sustainable in-house regulatory affairs team and mainly rely on outsourcing, thus creating lucrative opportunities for service providers.
In January 2021, Recipharm, a Sweden-based contract development and manufacturing organization (CDMO), formed a manufacturing agreement with a Sweden-based medical device startup, Enzymatica AB. Enzymatica AB outsourced manufacturing of its product, ColdZyme, a mouth spray against the common cold, to Recipharm.
Company Size Insights
The medium-sized companies segment led the market and accounted for a revenue share of 46.8% in 2020. The presence of several mid-sized established providers, especially privately held ones, is anticipated to contribute to this segment growth. These companies have a strong presence in selected markets or in multiple markets across the Nordic region. Their services may also vary from a few to a full range of services.
The large-sized companies segment is anticipated to register the fastest CAGR over the forecast period. They generally tend to prefer service providers of similar size to meet various regulatory needs arising due to their large geographic network and wide product lines and tend to seek long-term partnerships with service providers.
End-use Insights
The pharmaceutical companies segment dominated the market with a revenue share of 38.9% in 2020 and is projected to register the fastest CAGR from 2021 to 2028. This growth is attributed to the increase in the number of approved products of the leading pharmaceutical companies. Based on end-use, the market has been segmented into pharmaceutical, biotechnology, and medical device companies.
Biopharmaceutical companies are actively involved in the development of innovative molecules that fulfill the unmet needs of patients. With an increasing number of companies in the late-stage development of theirbiologics, the biologics market is anticipated to witness substantial growth in the future.
Service Insights
The regulatory writing and publishing segment held the largest revenue share of 36.8% in 2020. Pharmaceutical and biotech companies are increasingly opting for outsourcing selected regulatory functions, such as regulatory writing and publishing, to focus on core business needs. Based on service, the market is segmented into regulatory consulting, legal representation, regulatory writing and publishing, product registration and clinical trial applications, and others.
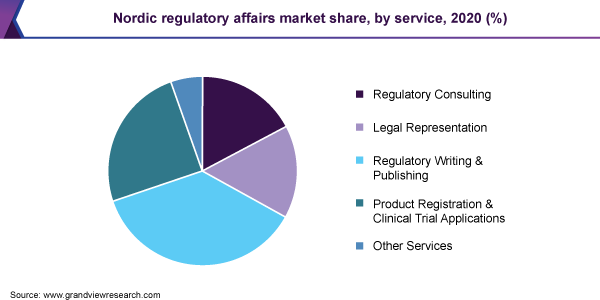
法律代表部分预计将register the fastest growth rate of 8.3% over the forecast period owing to the expansion plans of major biotech/pharma companies and medical devices companies in the Nordic region. For instance, in June 2020, Japan-based Fujifilm announced an investment of USD 1.0 billion to build a biologics plant in Denmark. Since this is an entry of a company from outside the Nordic region, the company will require the assistance of a legal representative. Legal representatives play a key role in bridging the gap between the difference in the local regulations and the entry of major pharma/biotech and medical device companies.
Country Insights
Sweden dominated the market in 2020 with a share of 59.6%. It is anticipated to witness the fastest growth over the forecast period owing to a rise in the number of drug development projects in the country. From an international perspective, it is shown that clinical trials performed in Sweden sites deliver superior quality results.
Despite a small population of almost 10 million, Sweden is known for its advanced healthcare system, willingness among the population to participate in research, and several collaborations with industry, academia, and healthcare organizations. Major pharma companies such as Pfizer, MSD, and GSK prefer Sweden for a significant number of their researches. Sweden is also included in GSK’s list of 9 EU countries, which it prefers for accelerating its clinical trial operations. In addition, Swedish clinics participate in 25% of all Pfizer global projects. Thus, Sweden serves as the global hub for drug development, eventually increasing the need for regulatory affairs in the country.
Key Companies & Market Share Insights
Companies provide various services such as regulatory consulting and writing and publishing for various pharmaceutical and medical devices companies. In January 2020, ICON Investments Limited acquired MedPass International, which has a significant presence in the European region as a regulatory and reimbursement consultant. The core competencies of MedPass are in market access and medical device development (class III). Some prominent players in the Nordic regulatory affairs market include:
Pharma Assist Sweden AB
GenPact Ltd.
ICON plc
Freyr
Global Pharma Consultancy AB
Regsmart Lifesciences AB
PRA Health Sciences
Charles River Laboratories International, Inc.
Parexel International Corporation, Inc.
Accell Clinical Research LLC
Nordic Regulatory Affairs Market Report Scope
Report Attribute |
Details |
Market Size value in 2021 |
USD 164.3 million |
Revenue forecast in 2028 |
USD 272.7 million |
Growth Rate |
CAGR of 7.5% from 2021 to 2028 |
Base year for estimation |
2020 |
Historical data |
2016 - 2019 |
Forecast period |
2021 - 2028 |
Quantitative units |
Revenue in USD million and CAGR from 2021 to 2028 |
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
Segments covered |
Service, service provider, company size, category, product stage, indication, end-use, country |
Regional scope |
Nordic |
Country scope |
Sweden; Norway; Denmark; Finland; Iceland |
Key companies profiled |
PRA Health Sciences; Charles River Laboratories International, Inc.; ICON plc; Parexel International Corporation, Inc.; Accell Clinical Research LLC |
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
革命制度党cing and purchase options |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
Segments Covered in the Report
This report forecasts revenue growth at the regional and country levels and provides an analysis of the latest industry trends and opportunities in each of the sub-segments from 2016 to 2028. For the purpose of this study, Grand View Research has segmented the Nordic regulatory affairs market report on the basis of service, service provider, company size, category, product stage, indication, end use, and country:
ServiceOutlook (Revenue, USD Million, 2016 - 2028)
Regulatory Consulting
Legal Representation
Regulatory Writing & Publishing
Product Registration & Clinical Trial Applications
Other Services
Service ProviderOutlook (Revenue, USD Million, 2016 - 2028)
In-house
Outsourcing
Company SizeOutlook (Revenue, USD Million, 2016 - 2028)
Small
Medium
Large
CategoryOutlook (Revenue, USD Million, 2016 - 2028)
Drugs
Biologics
Medical Devices
Product StageOutlook (Revenue, USD Million, 2016 - 2028)
Preclinical
Clinical Studies
PMA
IndicationOutlook (Revenue, USD Million, 2016 - 2028)
Oncology
Neurology
Cardiology
Immunology
Others
End-useOutlook (Revenue, USD Million, 2016 - 2028)
Medical Device Companies
Pharmaceutical Companies
Biotechnology Companies
CountryOutlook (Revenue, USD Million, 2016 - 2028)
Sweden
Norway
Denmark
Finland
Iceland
Frequently Asked Questions About This Report
b.The global Nordic regulatory affairs market size was estimated at USD 153.2 million in 2020 and is expected to reach USD 164.3 million in 2021.
b.The global Nordic regulatory affairs market is expected to witness a compound annual growth rate of 7.5% from 2021 to 2028 to reach USD 272.7 billion by 2028.
b.The regulatory writing & publishing held the largest share of 36.8% in 2020. Pharmaceutical and biotech companies are increasingly opting for outsourcing selected regulatory functions such as regulatory writing & publishing to focus on core business needs.
b.Some of the market players operating in the Nordic region include Pharma Assist Sweden AB, GenPact Ltd; PRA Health Sciences; Charles River Laboratories International, Inc.; ICON plc; Parexel International Corporation; Inc.; Freyr, Global Pharma Consultancy AB, Accell Clinical Research Llc; and Regsmart Lifesciences AB.
b.Changing regulatory landscape, increasing demand for the faster approval process, and economic & competitive pressures, are some of the key factors expected to drive the Nordic regulatory affairs market growth.





