
印第安纳州ia Ophthalmic Clinical Trials Market Size, Share & Trends Analysis Report By Product (Devices, Drugs/Pharmaceuticals), By Indication, By Phase, By Service Type, By Sponsor Type, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68040-096-7
- Number of Pages: 90
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- 印第安纳州ustry:Healthcare
Report Overview
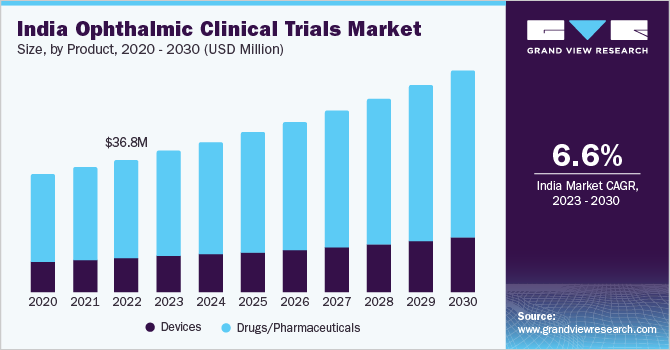
The印第安纳州ia ophthalmic clinical trials marketsizewas estimated atUSD 36.80 million in 2022and is expected to grow at a compound annual growth rate (CAGR) of 6.58% from 2023 to 2030. A favorable regulatory environment, rising demand for ocular treatment therapies, and government support are key growth drivers for the market. India has made a significant effort to streamline its regulatory framework forclinical trials, aiming to attract more research investment. Moreover, a substantial educated patient population pool along with healthcare facilities is anticipated to provide further growth opportunities for this market in the coming years.
The outbreak of COVID-19 had a major impact on theway clinical trials are administered and done in India due to the push for medical needs imposed by the pandemic. To prioritize resources for COVID-19 patients during the pandemic, numerous non-urgent or elective ophthalmic treatments, such as cataract surgeries and refractive surgeries, were delayed or canceled. This led to a reduction in the number of ophthalmic procedures performed, impacting the market revenue.
On the other hand, the increasing prevalence of eye disorders suchasglaucoma, dry eye disease, retinopathy, and cataract are expected to create growth opportunities for the ophthalmic clinical trials market. Glaucoma is the third most common reason for blindness in India. The affected population totals 12 million, or 12.8% of the nation's blindness.Glaucoma prevalence in India varies from 2.6% to 4.1%. Glaucoma-related blindness is permanent, and 50% of those who have it are unaware of it. Clinical studies are essential for expanding the understanding of glaucoma and creating novel techniques for its detection, treatment, and prevention.
Moreover, a favorable government environment is also anticipated to drive market growth in the forecast period. The implementation of initiatives like the establishment of ethical review committees has improved the effectiveness and transparency of the clinical trial approval procedure. Moreover, Indian governments have used a variety of strategies, such as targeted direct funding and industrial tax refunds, to try to increase the number of clinical trials in the country. This development contributed to the growth of the ophthalmic clinical trials industry in India.
Cost competitiveness is a significant factor that attracts players in the clinical trials sector to India. In India, clinical trial costs are one-tenth of the cost in the U.S. The amount spent on research and development in the pharmaceutical sector has increased dramatically due to numerous pharmaceutical corporations moving their clinical trial operations to India or outsourcing activities there. The regulatory organizations in India have established timelines to ensure the approval of trial applications in a comparatively short amount of time.
Product Insights
Based on product, the drugs/pharmaceuticals segment accounted for the largest market share of 73.90% in 2022. The growing prevalence of eye disorders and the increasing demand for effective treatment options have propelled pharmaceutical companies to invest significantly in ophthalmic drug development. Clinical trials are a critical step in the drug development process, enabling pharmaceutical companies to test the safety and efficacy of their ophthalmic products. India, with its large patient pool, diverse population, and well-established clinical research infrastructure, has emerged as an attractive destination for conductingophthalmic clinical trials. Thus, these factors are driving the growth of India’s ophthalmic clinical trials industry.
The devices segment is anticipated to register lucrative growth during the forecast period. Advancements in technology have led to the development of innovativeophthalmic devices, ranging from diagnostic tools to surgical instruments. These devices play a crucial role in the diagnosis, monitoring, and treatment of various eye disorders. Additionally, the growing demand for advanced ophthalmic devices and the increasing prevalence of eye diseases are driving the need for clinical trials in India.
Phase Insights
The clinical phase segment dominated the market in 2022 and accounted for the largest revenue share of 82.23%. The participation of pharmaceutical companies, research organizations, and healthcare institutions in different phases of clinical trials is driving the growth of the market. The diverse patient population in India allows for a comprehensive evaluation of the investigational products, ensuring their effectiveness across different genetic and environmental factors.
Furthermore, the preclinical phase segment is expected to grow at a substantial CAGR during the forecast period. The increasing emphasis on the safety and efficacy assessment of ophthalmic drugs and devices before proceeding to human trials has amplified the demand for robust pre-clinical studies. This segment plays a crucial role in identifying potential candidates, optimizing formulations, and assessing the safety profile of investigational products, thereby reducing the risk of adverse effects during subsequent clinical phases.
印第安纳州ication Insights
The retinopathy segment dominated the market for ophthalmic clinical trials with the largest revenue share of 24.89% in 2022. The increase in retinopathy-related research and development activities and the rising prevalence of retinopathy in the country are some of the factors driving the growth of the segment. According to a survey published by the Union Health Ministry in 2022, over 17% of Indians havediabetic retinopathy, with 3.6% of cases posing a potential threat to vision. A study has found that 10.4% of rural residents had diabetes, and about 10.3% had diabetic retinopathy. According to a WHO study, there would be a more than 50% increase in diabetics with retinal damage by the year 2030.
Furthermore, the glaucoma segment is expected to grow at a substantial CAGR during the forecast period. High prevalence of glaucoma, raising public awareness and knowledge of glaucoma are major factors propelling the market growth. The Indian government has recognized the burden of glaucoma and the requirement for ophthalmic research and development. The National Program for Control of Blindness and Visual Impairment is one initiative that aims to advance clinical research and eye care in the nation.
Service Type Insights
The clinical trial data managementservices segment dominated the India ophthalmic clinical trials market and accounted for the largest revenue share of 33.45% in 2022. Clinical trial data management services are crucial for ensuring the accuracy and integrity of data. They implement quality control measures and standardized processes to validate, collect, clean, and analyze the data generated during clinical trials.
Furthermore,bioanalytical testing services将举行一个重要的市场份额the coming years. Determining the drug release, distribution, and bioavailability in ocular tissues involves the use of bioanalytical testing services. This information aids in improving the design and development of ocular drug delivery systems for improved therapeutic results.
Sponsor Type Insights
The pharmaceutical/biopharmaceuticalcompanies dominated the market in 2022 and accounted for the largest share of 41.88 % of the revenue. The rising number of entities focusing on the R&D of novelophthalmic drugsis a major factor driving the market growth. Moreover, these entities offer a broad range of products, including ophthalmic therapies and drugs. Conducting clinical trials is an essential step in the development and approval process of new drugs, formulations, and treatment modalities.
另一段,包括横和医疗institutes is poised to register a lucrative CAGR during the forecast period. Medical institutes, including hospitals, universities, and research institutions, are centers of academic research in the field of ophthalmology. These institutions have specialized divisions and research facilities devoted to ocular studies. Clinical trials inside medical institutions are expanding due to advances in scientific understanding of eye illnesses and treatment options.
Key Companies & Market Share Insights
Key companies in the market undertake several strategic initiatives such as expansion, partnerships, new service launches, mergers, and acquisitions to strengthen their position in the market. For instance, in July 2021, Veeda Clinical Research announced the acquisition of Bioneeds India to expand its R&D services. This initiative was expected to expand the company’s service offering in the market. Some prominent players in the India ophthalmic clinical trials market include:
ProRelix Services LLP
Abiogenesis Clinpharm Pvt Ltd.
Veeda Clinical Research
Catawba Research, LLC
Novotech
Catalyst Clinical Services Pvt. Ltd.
Navitas Life Sciences
Vedic Lifesciences Pvt Ltd.
Vimta Labs Ltd
Others
印第安纳州ia Ophthalmic Clinical Trials Market Report Scope
Report Attribute |
Details |
Revenue forecast in 2030 |
USD 61.01 million |
Growth rate |
CAGR of 6.58% from 2023 to 2030 |
Base year for estimation |
2022 |
Historical data |
2018 - 2021 |
Forecast period |
2023 - 2030 |
Quantitative units |
Revenue in USD million, CAGR from 2023 to 2030 |
Report coverage |
收入预测,公司排名、竞争局域网dscape, growth factors, and trends |
Segments covered |
Product, service type, phase, indication, sponsor type |
Country scope |
印第安纳州ia |
Key companies profiled |
Abiogenesis Clinpharm Pvt Ltd; Veeda Clinical Research; Catawba Research, LLC.; Novotech; Catalyst Clinical Services Pvt. Ltd.; Navitas Life Sciences; Vedic Lifesciences Pvt Ltd.; Vimta Labs Ltd. |
Customization scope |
Free report customization (equivalent up to 8 analysts’ working days) with purchase. Addition or alteration to country, regional & segment scope. |
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
印第安纳州ia Ophthalmic Clinical Trials Market Report Segmentation
This report forecasts revenue growth and provides an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the India ophthalmic clinical trials market report based on product, services type, phase,sponsor type, and indication:
Product Outlook (Revenue, USD Million, 2018 - 2030)
Devices
Surgical Devices
Intraocular Lenses
Aspheric IOLs
Toric IOLs
Multifocal IOLs
Monofocal IOLs
Others
Others (Ophthalmic Lasers and other surgical devices)
Diagnostic Devices
Drugs/Pharmaceuticals
OTC Drugs
Prescription Drugs
印第安纳州ication Outlook (Revenue, USD Million, 2018 - 2030)
Macular Degeneration
Glaucoma
Dry Eye Disease
Retinopathy
Uveitis
Macular Edema
Blepharitis
Cataract
Optic Neuropathy
Others
Phase Outlook (Revenue, USD Million, 2018 - 2030)
Discovery Phase
Preclinical Phase
Clinical Phase
Phase I
Phase II
Phase III
Phase IV
Service Type Outlook (Revenue, USD Million, 2018 - 2030)
Protocol Designing
Site Identification
Patient Recruitment
Laboratory Services
Bioanalytical Testing Services
Clinical Trial Data Management Services
Others
Sponsor Type Outlook (Revenue, USD Million, 2018 - 2030)
Pharmaceutical/Biopharmaceutical Companies
Medical Device Companies
Others
Frequently Asked Questions About This Report
b.The India ophthalmic clinical trials market size was estimated at USD 36.80 billion in 2022 and is expected to reach USD 39.06 billion in 2023.
b.The India ophthalmic clinical trials market is expected to grow at a compound annual growth rate of 6.58% from 2023 to 2030 to reach USD 61.01 billion by 2030.
b.Drugs/Pharmaceuticals dominated the India ophthalmic clinical trials market with a share of 73.90% in 2022. This is attributable to growing prevalence of eye disorders and the increasing demand for effective treatment options have propelled pharmaceutical companies to invest significantly in ophthalmic drug development.
b.Some key players operating in the India ophthalmic clinical trials market include Abiogenesis Clinpharm Pvt Ltd, Veeda Clinical Research, Catawba Research, LLC., Novotech, Catalyst Clinical Services Pvt. Ltd., Navitas Life Sciences, Vedic Lifesciences Pvt Ltd., Vimta Labs Ltd.
b.Key factors that are driving the market growth include favorable regulatory environment, rising demand for ocular treatment therapies, and rising government support are some of the driving factors for the market.





