
Extractable And Leachable Testing Services Market Size, Share & Trends Analysis Report By Product (Container Closure Systems, Drug Delivery Systems), By Application (Parenteral Drug Products, OINDP), By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68040-112-9
- Number of Pages: 200
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry:Healthcare
Report Overview
The globalextractable and leachable testing services market sizewas valued atUSD 839 million in 2022and is expected to grow at a compounded annual growth rate (CAGR) of 15.13% from 2023 to 2030. The expanding pharmaceutical and biotechnology sectors across the world are fueling the demand for E&L testing services. Moreover, increasing regulatory scrutiny on the quality of healthcare products, the rise of complex drug formulations, such asbiologicsand combination products, and rising emphasis on product safety are further anticipated to propel the market growth.
The COVID-19 pandemic has positively impacted extractable and leachable testing services. With the heightened focus on healthcare and pharmaceuticals during the pandemic, there has been an increased awareness of the importance of rigorous testing to ensure the safety and efficacy of medical products. As a result, extractable and leachable testing services have gained more recognition and investment, leading to advancements in testing methodologies, equipment, and expertise. The pharmaceutical and medical device industries have been able to accelerate their research and development efforts to deliver innovative products while maintaining a strong commitment to safety and compliance. According to a WHO article, nearly 16 billion vaccine doses worth USD 141 billion were supplied in 2021, nearly thrice the market volume of 2019 (that is, 5.8 billion) and nearly three-and-a-half times the market value of 2019 (USD 38 billion). Thus, the increased demand for vaccines during the pandemic boosted the demand for extractable and leachable testing services.
The pharmaceutical andbiotechnologyindustry has been rapidly growing in recent years due to advancements in technology, increased demand for novel drugs, and a growing incidence & prevalence of chronic diseases. This has also led to increased R&D activities for the creation of novel therapeutics. For instance, in 2022, the U.S. FDA’s Center for Drug Evaluation and Research (CDER) approved 37 new drugs, either as new molecular entities or as new therapeutic biological products. Thus, high product development and commercialization by pharmaceutical industries created a higher demand for extractable and leachable testing services.
Furthermore, patient safety is the top priority for medical devices and pharmaceutical industries and they are making significant efforts to safeguard the final product. Pharmaceutical companies are investing largely to develop robust methods for leachable and extractable testing. According to Merck KGaA, leachable and extractable are compounds that can migrate from the container to the formulation and can produce serious adverse effects such as toxicity and adverse drug reactions. To mitigate this, the company developed its pre-qualified secondary Certified Reference Materials (CRMs) and ready-to-use CRM mixtures. This reference material is certified ISO 17025 and 17034. This guidance material from the company can act as SOP for analytical laboratories providing extractable & leachable testing services. Thereby increasing the demand for extractable and leachable testing services over the forecast period.
此外,监管机构的存在,such as the FDA and the U.S. Pharmacopeia (USP), is pushing manufacturers to test their products for E&L substances. In addition, the rising use of single-use technologies for scaling up the process and various strategic initiatives undertaken by market players will likely create lucrative growth opportunities for the market. For instance, in May 2022, Pacific BioLabs launched In Vitro Services to support the biopharma, pharmaceuticals, and medical device industries. This service aided in the expansion of the company’s testing capabilities in analytical/bioanalytical and in vivo departments. Hence, the above-mentioned factors support the country’s market.
Product Type Insights
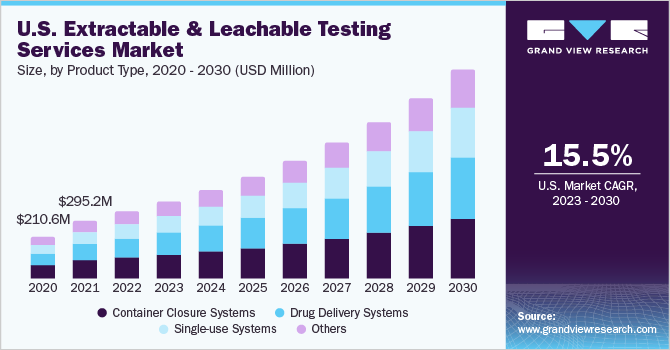
On the basis of product type, the container closure systems segment held the largest revenue share of 31% of the market. The revenue share of container closure systems (CCS) is dominating due to their capability to offer long-term stability in formulations. CCS includes various package and delivery systems, which can leach substances over time, making extractable and leachable (E & L) testing crucial. Ensuring adequate container closure systems is vital to uphold the safety, sterility, and overall quality of drugs. Several instances have been noted where the safety of pharmaceutical products was compromised, or harmful contaminants formed as a result of impurities transferring from container closure systems to the drugs. Regulatory agencies have released guidelines to govern container closure systems and packaging materials in the pharmaceutical industry. For instance, the Food and Drug Administration (FDA) provided guidance in the Code of Federal Regulations Title 21, which emphasizes that container closure systems must safeguard drug products from potential external factors that could lead to contamination or degradation during storage and use.
The single-use systems segment is expected to grow at a significant growth rate of 16.87% during 2023-2030. The use of single-use components and systems in commercial and clinical biopharmaceutical manufacturing has witnessed a substantial rise. These components are typically composed of polymers orplastics. Single-use systems offer numerous advantages, such as enhanced speed, flexibility, and operational efficiency compared to reusable components. However, a significant concern associated with these single-use systems is the potential leaching of compounds from polymeric materials, which can negatively affect the quality of pharmaceutical products or the efficiency of the manufacturing process. Consequently, the importance of extractable and leachable testing has grown significantly to address this issue for single-use systems.
Application Insights
The orally inhaled and nasal drug products (OINDP) segment dominated the market with a market share of 41.88% in 2022. This large share can be attributed to the growing service launches for this high-risk category of drugs and the presence of guidelines and best practices for the E&L testing of OINDP. Orally inhaled and nasal drug products include nasal sprays, metered dose inhalers, dry powder inhalers,nebulizers, and inhalers. These products are widely used for systemic delivery of various therapeutics. Thus, increasing the demand for extractable and leachable testing services for this segment over the forecast period.
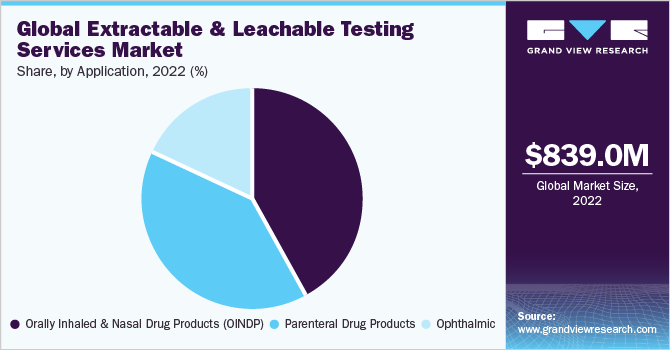
The parenteral drug products segment is projected to witness a significant growth rate of 17.24% from 2023 to 2030. Parenteral drugs are administered into the muscles, veins, or specialized tissues, such as the spinal cord or under the skin. The extractable & leachable testing of parenteral drug products of injectable helps ensure efficacy and safety of parenteral drug products. Thus companies, authorities, and regulatory bodies emphasize extractable and leachable testing for parenteral drugs. They are undertaking initiatives to enhance awareness about E&L testing and its applications for parenteral drug products. For instance, in August 2021, the Parenteral Drug Association presented a webinar series for extractable and leachable parenteral applications. This webinar covered various topics, including analytical E/L methodologies and large-volume parenteral. Moreover, in October 2021, the Parenteral and Ophthalmic Drug Products (PODP) L&E Working Group of the Product Quality Research Institute (PQRI) presented recommendations for E&Ls in parenteral drug products (PDP) to the US Food and Drug Administration (FDA). Thus, extractable and leachable testing is essential for parenteral drug products to improve the quality of drugs.
Regional Insights
43岁的北美拥有最大的市场份额。71% in 2022. The significant share of this region in the market is primarily due to the increasing adoption of novel technologies and biopharmaceuticals for the analysis and treatment of clinical conditions. Moreover, a large number of market players in the region are actively involved in continuously improving advanced tools for biopharmaceutical research. Additionally, government funding for research has played a crucial role in boosting the extractable and leachable testing services market. For instance, in June 2021, the U.S. Department of Agriculture’s (USDA) National Institute of Food and Agriculture (NIFA) invested USD 5.4 million in research related to bioengineering, bioprocessing, biofuels, and other biobased products, further supporting the growth of the industry.
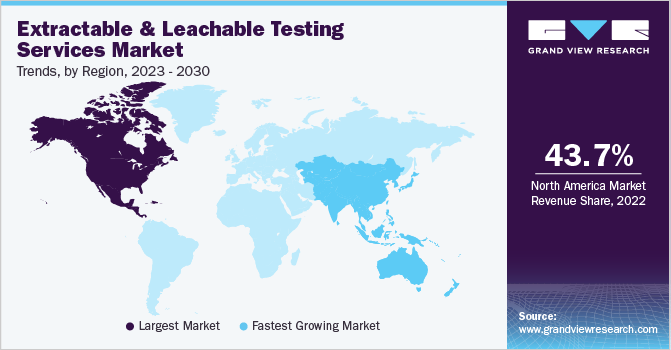
The Asia Pacific region is anticipated to have a significant CAGR of 15.32% during the forecast period. The notable advancements in the pharmaceutical andbiotechnologysectors in the region are key drivers propelling the growth of single-use technology. Specifically, countries like China, India, and Japan are expected to experience substantial expansion, due to increased government investments in research and development centers. This, in turn, is driving the widespread adoption of single-use systems in vaccine production and various other research & therapy areas. For instance, in March 2023, Hetero Drugs made a USD 122 million investment in Andhra Pradesh to grow the state's pharmaceutical sector. Furthermore, according to statistics from the state industrial division, Andhra Pradesh's pharma sector garnered around USD 2 billion in investment in 2022.
Key Companies & Market Share Insights
Market leaders are involved in extensive R&D for manufacturing cost-efficient and technologically advanced testing products and services. Several strategies, such as mergers & acquisitions, undertaken by these organizations to expand their market presence are anticipated to create significant growth opportunities over the forecast period. For instance, in March 2023, Nelson Laboratories, LLC – A Sotera Health Europe collaborated with Nemera, adrug delivery devicesolutions provider, to offer integrated services including E & L services to their customers. Some of the key players operating in the global extractable and leachable testing services market include:
Eurofins Scientific
Intertek Group plc
SGS Société Générale de Surveillance SA
WuXi AppTec
Merck KGaA
West Pharmaceutical Services, Inc
Wickham Micro Limited (Medical Engineering Technologies Ltd.)
Pacific Biolabs
Boston Analytical
Sotera Health (Nelson Laboratories, LLC)
Extractable And Leachable Testing Services MarketReport Scope
Report Attribute |
Details |
Market size value in 2023 |
USD 959.5 million |
Revenue forecast in 2030 |
USD 2.57 billion |
Growth Rate |
CAGR of 15.13% from 2023 to 2030 |
Base year for estimation |
2022 |
Historical data |
2018 - 2021 |
Forecast period |
2023 - 2030 |
Quantitative units |
Revenue in USD Million and CAGR from 2023 to 2030 |
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
赛格ments covered |
Product type, application, and region |
Regional scope |
North America, Europe, Asia Pacific, Latin America, MEA |
Country scope |
U.S., Canada, UK, Germany, France, Italy, Spain, Norway, Sweden, Denmark, Japan, China, India, Australia, South Korea, Thailand, Brazil, Mexico, Argentina, South Africa, Saudi Arabia, UAE, Kuwait |
Key companies profiled |
Eurofins Scientific, Intertek Group plc, SGS Société Générale de Surveillance SA, WuXi AppTec, Merck KGaA, West Pharmaceutical Services, Pacific Biolabs, Medical Engineering Technologies Ltd., Boston Analytical, Sotera Health (Nelson Laboratories, LLC) |
Customization scope |
Free report customization (equivalent up to 8 analysts’ working days) with purchase. Addition or alteration to country, regional & segment scope |
Global Extractable And Leachable Testing Services MarketReport赛格mentation
This report forecasts revenue growth and provides an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global extractable and leachable testing services market based on product type, application, and region:
Product Type Outlook (USD Million; 2018 - 2030)
Container Closure Systems
Single-use Systems
Drug Delivery Systems
Others
Application Outlook (USD Million; 2018 - 2030)
Parenteral Drug Products
Orally Inhaled and Nasal Drug Products (OINDP)
Ophthalmic
Regional Outlook (USD Million, 2018 - 2030)
North America
U.S.
Canada
Europe
UK
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
Australia
South Korea
Thailand
Latin America
Brazil
Mexico
Argentina
MEA
South Africa
Saudi Arabia
UAE
Kuwait
Frequently Asked Questions About This Report
b.The global extractable and leachable testing services market size was estimated at USD 839.0 million in 2022 and is expected to reach USD 959.5 million in 2023.
b.The global extractable and leachable testing services market is expected to grow at a compound annual growth rate of 15.13% from 2023 to 2030 to reach USD 2.57 billion by 2030.
b.In 2022, the orally inhaled and nasal drug products (OINDP) segment dominated the market with a market share of 41.88%. This dominance can be attributed to the growing service launches for this high-risk category of drugs and the presence of guidelines and best practices for the E&L testing of OINDP.
b.Some of the key players operating in the global extractable and leachable testing services market include Eurofins Scientific, Intertek Group plc, SGS Société Générale de Surveillance SA, WuXi AppTec, Merck KGaA, West Pharmaceutical Services, Pacific Biolabs, Medical Engineering Technologies Ltd., Boston Analytical, and Sotera Health (Nelson Laboratories, LLC).
b.The expanding pharmaceutical and biotechnology sectors across the world are fueling the demand for E&L testing services. Moreover, increasing regulatory scrutiny on the quality of healthcare products, the rise of complex drug formulations, such as biologics and combination products, and rising emphasis on product safety are further anticipated to propel the market growth.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."






