
External Defibrillators Market Size, Share & Trends Analysis Report By Product (Manual External Defibrillators, Automated External Defibrillators, Wearable Cardioverter Defibrillators), By End Use, By Region, And Segment Forecasts, 2022 - 2030
- Report ID: GVR-4-68039-831-1
- Number of Pages: 140
- Format: Electronic (PDF)
- Historical Range: 2017 - 2020
- Industry:Healthcare
Report Overview
The global external defibrillators market size was valued at USD 2.9 billion in 2021 and is estimated to expand at a compound annual growth rate (CAGR) of 9.3% from 2022 to 2030. Increasing technological advancements, government initiatives for installation of AEDs at public places, incidence of sudden cardiac arrest, and initiatives by key market players are some of the key drivers of this market. In November 2020, OHS Canada reported that the Defibrillator Registration and Public Access Act (Bill 141) was accepted by the government and is anticipated to increase public access to AEDs in the country. The COVID-19 pandemic significantly boosted the demand for External Defibrillators in spite of the supply chain challenges and other hurdles brought by movement restrictions.
Nihon Kohden for instance, reported double-digit growth in international sales in 2020 (including the U.S. and Latin America) due to the increased demand during the COVID-19 pandemic. Sales in Mexico and Colombia, in particular, recorded 200% growth due to surge in demand for Nihon Kohden’s patient monitors, defibrillators, and ventilators. In June 2020, Philips received FDA clearance for HeartStartFRx and HeartStart FR3 automated external defibrillators. HeartStart FR3 includes innovative features to help medical personnel and first-responders treat cardiac arrest. While HeartStartFRx is a public-access automated external defibrillators that features intuitive, step-by-step voice instructions, comprising cardiopulmonary resuscitation guidance, for emergency use in school, workplaces, and other public spaces, in addition to medical professional use.
Increasing number of supportive policies by governments, regulatory bodies, and healthcare organizations is anticipated to fuel the market over the forecast period. For example, the IRC (Italian Resuscitation Council) reported that the proposed law on AEDs in Italy was approved by the Social Affairs Commission of the Chamber in July 2021. The law provides for about 10 million EUR (about USD 11.36 million) for the implementation of public access AEDs over the next five years, among other provisions. In Australia, the government agency SA Ambulance Service (SAAS), recommends use of a defibrillator as early as possible to increase the person’s chances of survival. Both semiautomatic and automatic AEDs are recommended while manual defibrillators are recommended for use by trained professionals only. AEDs are also recommended over a manual defibrillator in low acuity settings, including medical clinics and allied health and dental clinics, due to their ease of use.
技术进步导致了现代延期rnal defibrillators becoming more and more compact and easier to use, with reduced need for maintenance and increased cost-effectiveness. AEDs in the U.S., for instance, are priced between USD 900 and 2,000. AED USA offers the Zoll AED Plus Fully Automatic defibrillators at USD 1,699 and the ZOLL AED three semi-automatic at USD 1,895. Prices of manual external defibrillators, on the other hand, range from USD 1,000 to 25,000 according to the WHO. Emerging technology trends in the market are advancements in personal AEDs for home use, use of drones to improve cardiac arrest response, solutions to improve tracking of AEDs, and use of IoT and analytics. Rapid Response Revival Research Ltd, based in Australia, has developed the world’s first personal AED-CellAED-for home use. The device received CE Certification in May 2021, enabling the company to commercialize the device across Europe.
Product Insights
The manual external defibrillators segment dominated the market and accounted for the largest revenue share of more than 39.0% in 2021. The AEDs segment on the other hand is estimated to witness the fastest CAGR of 9.4% over the forecast period. This is because of growing initiatives by major companies, increasing adoption of defibrillators at healthcare facilities, and deployment of several public access defibrillation programs. Mindray, a key market player, has deployed hundreds of its BeneHeart AED and manual defibrillators across the local health board of NHS Wales-BetsiCadwaladr University Health Board in the U.K. This enabled the BetsiCadwaladr University Health Board to standardize the resuscitation equipment in hospitals for the entire North Wales region in compliance with the Resuscitation Council (U.K.) guidelines.
End-use Insights
By end use, the hospitals segment held the largest revenue share of over 25.0% in 2021. This is attributable to higher volume of patients received in hospitals, growing adoption of external defibrillators by hospitals, and strategies implemented by key manufacturers. The cardiology units in the hospitals require defibrillators to handle patients facing cardiac-related problems. Rising incidence of hypertension, diabetes, and strokes leading to SCA due to lack of physical activity, unhealthy dietary habits, and smoking is likely to drive demand for defibrillators in hospitals. Zoll, a part of Asahi Kasei, reported that it held the largest share of the defibrillators market used for hospitals and EMS in November 2021.
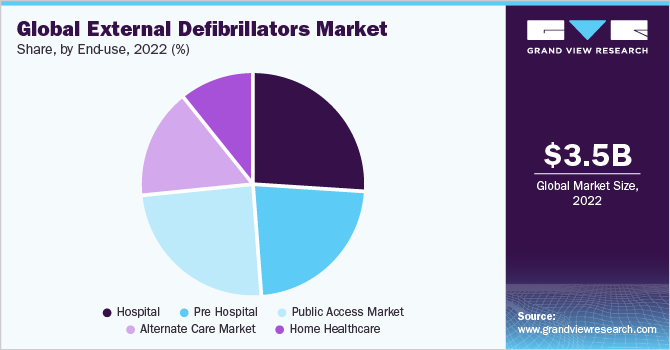
The public access segment is projected to witness the fastest CAGR of 9.6% over the forecast period owing to supportive initiatives by governments, regulatory agencies, and healthcare institutions to increase the availability of public access AEDs. The Automated External Defibrillators (Public Access) Bill was introduced in the House of Commons of the U.K. Parliament, in June 2021. If the bill receives royal assent, it would become mandatory to install defibrillators at public places such as sporting facilities, schools, skills facilities, buildings, and facilities that provide care to vulnerable people.
Regional Insights
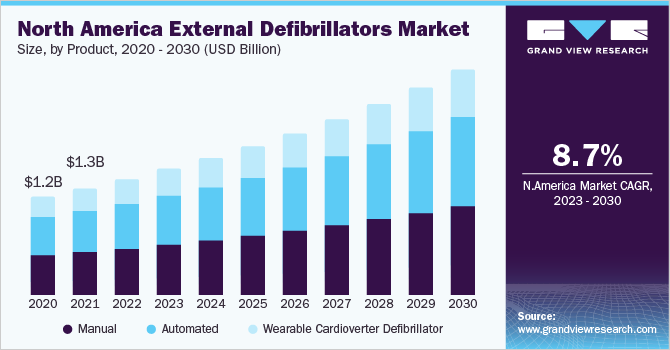
North America held a revenue share of more than 43% in 2021. The large share of the North American region can be attributed to the presence of key players, supportive regulations to increase the availability of public access AEDs, and the high adoption of external defibrillators at healthcare facilities. Many national organizations in the U.S., including the American Heart Association (AHA), recommend the implementation of comprehensive Public Access Defibrillation (PAD) programs. The suggested attributes for such a program include targeted placement of AEDs (including their routine maintenance and testing), imparting training to responders, Emergency Medical Services (EMS) coordination, and continuous quality improvement.
Europe is estimated to witness the fastest CAGR of 10.2% in the external defibrillators market over the next few years. This is owing to well-established healthcare infrastructure, large geriatric population susceptible to cardiovascular adverse events, and local presence of key companies. For instance, WEINMANN Emergency Medical Technology GmbH + Co. KG is a German manufacturer that offers the MEDUCORE Standard series of external defibrillators for resuscitation and patient monitoring. The series is intended to be used by emergency medical services personnel, military medical corps, and hospitals.
Key Companies & Market Share Insights
The market for external defibrillators is competitive in nature. The market is highly fragmented, owing to the presence of several small as well as large players. This results in high competition among smaller players looking to sustain their position in the market. Moreover, companies are increasingly adopting strategies such as mergers and acquisitions, geographic expansion, and launch of new products to grow in the market. For instance, in April 2019 Stryker launched LIFEPAK CR2 AED in the U.S., expanding its lineup of defibrillation solutions. The product connects to an AED program manager, LIFELINKcentral, to help an organization’s AED manager to monitor and manage device readiness issues remotely. When connected to Wi-Fi, the device also provides near real-time email alerts. Some of the key players in the external defibrillators market include:
Koninklijke Philips N.V.
Stryker
Zoll Medical Corporation, an Asahi Kasei Group Company
Nihon Kohden Corporation
ProgettiSrl
Schiller AG
MS Westfalia GmbH
AMI Italia
Bexen Cardio
Silverline Meditech Pvt. Ltd.
Mediana Co., Ltd.
Shenzhen Mindray Bio-Medical Electronics
CU Medical
BPL Medical Technologies
Corpuls
Dixion Vetrieb medizinischer Geräte GmbH
Bioevopeak Co., Ltd.
METsis Medikal
EMS Mobil Sistemler A.Ş.
Metrax GmbH
External Defibrillators Market Report Scope
Report Attribute |
Details |
Market size value in 2022 |
USD 3.2 billion |
Revenue forecast in 2030 |
USD 6.5 billion |
Growth Rate |
CAGR of 9.3% from 2022 to 2030 |
Base year for estimation |
2021 |
Historical data |
2017 - 2020 |
Forecast period |
2022 - 2030 |
Quantitative units |
Revenue in USD million and CAGR from 2022 to 2030 |
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
Segments covered |
Product, end-use, and region |
Regions covered |
北美;欧洲;亚太地区;拉丁美洲; MEA |
Country Scope |
U.S.; Canada; U.K.; Germany; Italy; France; Spain; Netherlands; Japan; China; India; Australia; South Korea; Brazil; Mexico; Argentina; Colombia; South Africa; Saudi Arabia; UAE |
Key companies profiled |
Koninklijke飞利浦喷嘴速度;Stryker;海关医疗有限公司rporation (Asahi Kasei Group); Nihon Kohden Corporation; ProgettiSrl; Schiller AG; MS Westfalia GmbH; AMI Italia; Bexen Cardio; Silverline Meditech Pvt. Ltd.; Mediana Co., Ltd.; Shenzhen Mindray Bio-Medical Electronics; CU Medical; BPL Medical Technologies; Corpuls; Dixion Vetrieb medizinischer Geräte GmbH; Bioevopeak Co., Ltd.; METsis Medikal; EMS Mobil Sistemler A.Ş.; Metrax GmbH |
Customization scope |
Free report customization (equivalent up to 8 analysts’ working days) with purchase. Addition or alteration to country, regional, and segment scope. |
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
Segments Covered in the Report
这份报告预测全球收入增长, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2017 to 2030. For the purpose of this report, Grand View Research has segmented the global external defibrillators market report on the basis of product, end-use, and region:
Product Outlook (Revenue, USD Million, 2017 - 2030)
Manual External Defibrillators
Automated External Defibrillators
Semi-Automated ED
Fully Automated ED
Wearable Cardioverter Defibrillators
End-use Outlook (Revenue, USD Million, 2017 - 2030)
Hospital
Pre Hospital
Public Access Market
Alternate Care Market
Home Healthcare
Regional Outlook (Revenue, USD Million, 2017 - 2030)
North America
U.S.
Canada
Europe
U.K.
Germany
France
Italy
Spain
Netherlands
Asia Pacific
Japan
China
India
Australia
South Korea
拉丁美洲
Brazil
Mexico
Argentina
Colombia
Middle East & Africa
South Africa
Saudi Arabia
UAE
Frequently Asked Questions About This Report
b.The global external defibrillators market size was estimated at USD 2.9 billion in 2021 and is expected to reach USD 3.2 billion in 2022.
b.The global external defibrillators market is expected to grow at a compound annual growth rate of 9.3% from 2022 to 2030 to reach USD 6.5 billion by 2030.
b.North America dominated the external defibrillators market with a share of 43% in 2021. This is attributable to the presence of key players and supportive regulatory policies.
b.Some key players operating in the external defibrillators market include Koninklijke Philips N.V.; Stryker; ZOLL Medical Corporation; Nihon Kohden Corporation; Progetti Srl; Schiller AG; MS Westfalia GmbH; AMI Italia; Bexen Cardio; Silverline Meditech Pvt. Ltd.; Mediana Co., Ltd.; Shenzhen Mindray Bio-Medical Electronics; CU Medical; BPL Medical Technologies; and Corpus.
b.Key factors that are driving the external defibrillators market growth include rising technological advancements, initiatives for promoting public access AEDs, and incidence of sudden cardiac arrest.





