
eConsent In Healthcare Market Size, Share & Trends Analysis Report By Platform (Cloud-based, Web-based), By Enrollment Type, By Form Type, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68040-078-5
- Number of Pages: 150
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry:Healthcare
Report Overview
The globaleConsent in healthcare market sizewas valued atUSD 392.29 million in 2022and is expected to grow at a compound annual growth rate (CAGR) of 11.2% from 2023 to 2030. The increasing adoption of eConsent in clinical trials, aided by mobile technology advancements that have revolutionized the process of informed consent and improved patient comprehension, is expected to drive growth in the eConsent market within the healthcare industry over the forecast period.
For instance, according to the “14 Drivers of eConsent Adoption in Clinical Trials - Infographic” published by Signant Health, leading pharmaceutical companies are investing in the adoption of eConsent in their clinical trial systems. The document states that 100% of the top 10 pharmaceutical companies have implemented eConsent initiatives, while 88% of the top 25 pharmaceutical companies have also implemented eConsent. In addition, 66% of the top 50 pharmaceutical companies are currently engaged or planning to initiate an eConsent program in the near future.
Furthermore, market growth is bolstered by the healthcare industry's emphasis on streamlining processes and reducing reconciliation through eConsent integration with othereClinical solutions, as well as a surge in strategic acquisitions & partnership deals. For instance, in June 2022, uMotif & ClinOne partnered to provide a single, integrated solution that delivers best-in-class electronic informed consent (eConsent) and electronic clinical outcome assessments technology to tackle the challenges faced by patients & clinical research coordinators in navigating multiple systems, apps, & sensors for each protocol in the life sciences industry.
COVID-19大流行有积极的影响global eConsent market by accelerating the adoption of eConsent worldwide through regulatory flexibility. In particular, Europe made significant progress in implementing eConsent, with regulatory authorities actively developing recommendations and document drafting in multiple regions.For instance, Medidata Solutions' whitepaper “Electronic Informed Consent in Clinical Research” stated that the pandemic led to a more flexible acceptance of technologies, such as eConsent, which enable trial continuation while mitigating infection risk.
Furthermore, eConsent has been identified as an acceptable alternative during the COVID-19 pandemic in certain clinical trial guidance documents. The global lack of regulatory clarity on eConsent has been a longstanding issue, but progress is being made. For instance, draft ICH GCP E6 (R3) references “the potential use of technology to inform participants and obtain informed consent.” However, eConsent adoption has been variable in Europe due to a lack of regulatory clarity and operational issues, such as the acceptance of electronic signatures for eConsent documents.
Platform Insights
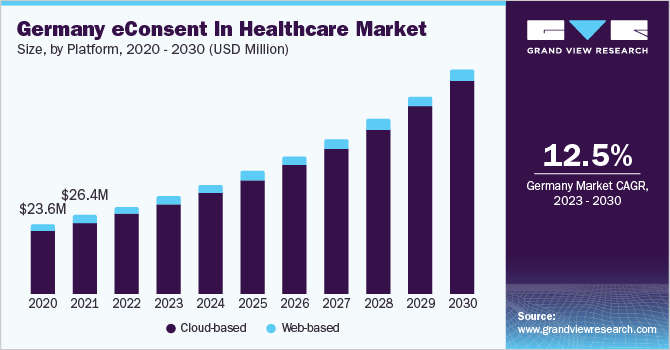
In 2022, the cloud-based segment gained a major market share of over 90%. The segment growth can be attributed to the increasing usage of cloud-based platforms due to the advantage of reducing administrative burden and ensuring compliance for sites & study teams. Besides, cloud-based eConsent enhances the process with the deployment of modern technology and multimedia to create an interactive informed consent experience for potential trial participants. It further adds immediate value for customers, accelerates timelines and maintains study budgets.
此外,基于云计算平台节省时间clinical trials with multiple sites of patient enrollment, remotely monitored participants, and other large, randomized studies. It also supports data aggregation into a central repository, significantly reducing the duration of a clinical trial. For instance, in November 2022, YPrime, LLC launched an eConsent platform that helps study teams present complex patient information in digestible components, making it highly navigable and improving understanding and engagement. This platform is expected to streamline the clinical trial process and improve patient recruitment and retention.
On the other hand, web-based eConsent is based on obtaining information through a website or web-based application. It allows sites to obtain information that is not available over a paper process. This consent is made accessible online, hence users can access it as part of their website usage and get an online experience.
Enrollment Type Insights
The onsite segment dominted the market and accounted for revenue share of over 80% in 2022. Onsite enrollment refers to participants providing consent for a study in person using the eConsent platform in a clinical setting. The growth of this segment is driven by the increasing adoption of eConsent in clinical trials and the demand for technologically advanced platforms to boost productivity. Additionally, eConsent simplifies the administrative process and minimizes site visits. Onsite enrollment has led to an increase in eConsent usage for disease registries, clinical study consents, and biobanking registries in healthcare. These factors have contributed to the rising market demand for eConsent solutions.
However, remote enrollment is anticipated to witness the fastest growth over the forecast period. Remote enrollment for screening or clinical trials can be facilitated through standalone eConsent platforms or integrated eClinical solutions. Furthermore, the launch of new platform solutions that support eConsent/re-consent for remote, traditional, or hybrid trials are anticipated to further support market growth. For example, in April 2021, Signant Health expanded its electronic informed consent offerings with the release of SmartSignals eConsent. This offering supports global eConsent/re-consent for various types of trials such as remote, traditional, or hybrid trials.
Form Type Insights
The general consent forms held the highest share in 2022, accounting for over 35%. These forms are widely adopted in healthcare facilities and clinical trials due to their simplicity compared to other forms. General consent forms do not require detailed information about past healthcare experiences, reducing the burden of maintaining patient records. They are typically used for patient care processes that do not pose significant risks, such as collecting blood samples, physical examinations, or administering fluids.
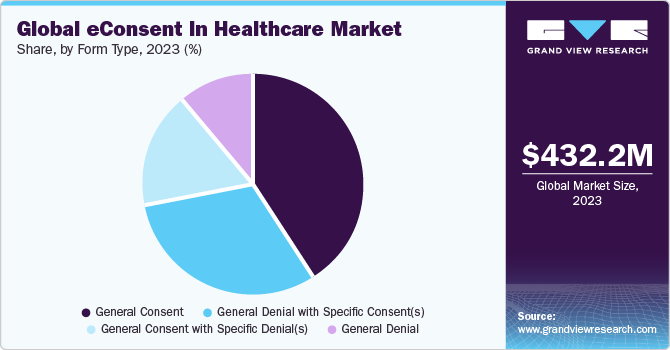
On the other hand, General denial with specific consent(s) is anticipated to witness growth at a lucrative growth rate over the estimated time period. This form is specifically created for patients who want to restrict access to their health data, except in specific situations outlined in the consent. For instance, a patient may authorize their primary care provider to send a body fluid sample to a diagnostic service with only the necessary data for conducting tests and reporting results.
Regional Insights
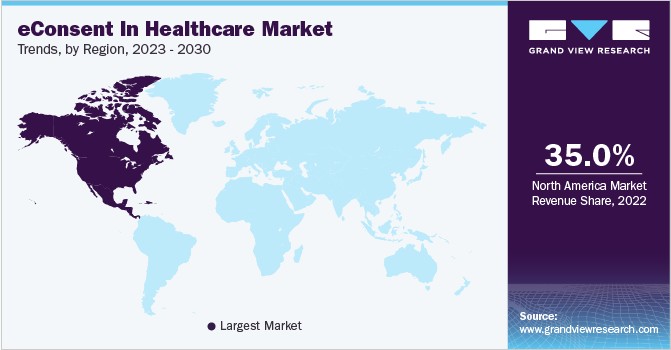
In 2022, North America held the largest market share of over 35%. The region has witnessed successful widescale implementation of eConsent in healthcare, driven by the high demand and growing digitalization of patient health information. The adoption of advanced eConsent solutions is essential for improving healthcare outcomes and supporting clinical trials in the region. The market has also benefited from the increased need for remote data collection and government initiatives during the COVID-19 pandemic. Government support in the U.S. and Canada further fuels the adoption of eConsent processes and contributes to the growth of the regional market.
The eConsent in healthcare market in Europe is driven by various factors, including healthcare regulations, the growing patient population, the rise in clinical trials, the impact of the COVID-19 pandemic, and regulations surrounding electronic informed consent. Several European countries are also implementing eConsent solutions by introducing new updates and features. In March 2021, the European Contract Research Organisation Federation (EUCROF) published a guide on electronic informed consent implementation, highlighting that the general eIDAS regulation on eSignatures lacks specific guidance for using eSignatures in clinical studies. These developments contribute to the growth and advancement of the eConsent market in Europe.
In the Asia Pacific region, the demand for eConsent in Healthcare is increasing across multiple countries due to the rising disease burden, rapid economic development, and growing adoption of technology-based solutions, including cloud and web-based platforms, in clinical trials. Moreover, there is a strong focus on research and clinical trials in hospitals and research centers, leading to an increased need for digital solutions like eConsent. An example is the selection of Signant Health in September 2019 to provide electronic informed consent for a neuroscience study conducted by the Shanghai Mental Health Center (SMHC). This study, which enrolled hundreds of patients at various sites, marked the initial implementation of Signant's Trial Consent solution in China. These developments highlight the emerging requirement and adoption of eConsent solutions in the Asia Pacific for improved clinical trial processes and patient engagement.
Key Companies & Market Share Insights
The global market for e-Consent in healthcare is highly competitive, with notable players such as Veeva Systems, Wellbeing Software, a Citadel Group, Catalyst EMR and Cloud BYZ, among others. These companies are constantly engaged in launching new products, conducting clinical trials, and other related developments to stay ahead of the competition.
For example, in October 2020, Veeva Systems announced the launch of two new applications built on the Veeva Clinical Network - Veeva Site Connect and Veeva eConsent. Veeva Site Connect facilitates paperless information exchange between clinical research and sponsor sites throughout a trial. With Veeva eConsent, researchers can digitize the consent process with review boards and patients while ensuring transparency for sponsors. Some of the prominent players in the global eConsent in healthcare market include:
Veeva Systems
Wellbeing Software, a Citadel Group
Florence Healthcare
Concentric Health Ltd
5thPort, LLC
Cloudbyz
Calysta EMR
Interlace Health
Thieme Compliance GmbH
eConsent In Healthcare Market Report Scope
Report Attribute |
Details |
Market size value in 2023 |
USD 432.15 million |
Revenue forecast in 2030 |
USD 909.14 million |
Growth rate |
CAGR of 11.2% from 2023 to 2030 |
Base year for estimation |
2022 |
Historical data |
2018 - 2021 |
Forecast period |
2023 - 2030 |
Quantitative units |
Revenue in USD million and CAGR from 2023 to 2030 |
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
Segments covered |
Platform, enrollment type, form type, region |
Regional scope |
北美;欧洲;亚太地区;拉丁美洲; MEA |
Country scope |
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; Thailand; South Korea; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE |
Key companies profiled |
Veeva Systems; Wellbeing Software; a Citadel Group; Florence Healthcare; Concentric Health Ltd; 5thPort, LLC; Cloudbyz; Calysta EMR; Interlace Health; Thieme Compliance GmbH |
Customization scope |
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope. |
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
GlobaleConsent In Healthcare Market ReportSegmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this report, Grand View Research has segmented the global eConsent in healthcare market report on the basis of platform, enrollment type, form type, and region:
Platform Outlook (Revenue, USD Million, 2018 - 2030)
Cloud-based
Web-based
Enrollment Type Outlook (Revenue, USD Million, 2018 - 2030)
On-site
Remote
Form Type Outlook (Revenue, USD Million, 2018 - 2030)
General Consent
General Consent with Specific Denial(s)
General Denial with Specific Consent(s)
General Denial
Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
U.S.
Canada
Europe
UK
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
Australia
Thailand
South Korea
拉丁美洲
Brazil
Mexico
Argentina
Middle East & Africa
South Africa
Saudi Arabia
UAE
Frequently Asked Questions About This Report
b.The global eConsent in Healthcare market size was estimated at USD 392.29 million in 2022 and is expected to reach USD 432.15 million in 2023.
b.The global eConsent in Healthcare market is expected to grow at a compound annual growth rate of 11.2% from 2023 to 2030 to reach USD 909.14 million by 2030.
b.North America dominated the eConsent in Healthcare market with a share of 39.2% in 2022. This is attributable to its adoption of eClinical solutions, including eConsent platforms, which have reduced clinical process-related time and improved clinical trial process efficiencies.
b.Some key players operating in the eConsent in Healthcare market include Veeva Systems, Wellbeing Software, a Citadel Group, Florence Healthcare, Concentric Health Ltd, 5thPort, LLC, Cloudbyz, Calysta EMR, Interlace Health, Thieme Compliance GmbH.
b.关键因素推动了eConsent Healthcare market growth include the rising adoption of eConsent in clinical trials, aided by mobile technology advancements that have revolutionized the process of informed consent and improved patient comprehension. Furthermore, market growth is bolstered by the healthcare industry's emphasis on streamlining processes and reducing reconciliation through eConsent integration with other eClinical solutions and a surge in strategic acquisitions & partnership deals.





