
Drug Device Combination Products Market Size, Share & Trends Analysis Report By Product (Transdermal Patches, Inhalers, Infusion Pumps, Drug Eluting Stents, Antimicrobial Catheters), And Segment Forecasts, 2022 - 2030
- Report ID: GVR-1-68038-260-0
- Number of Pages: 105
- Format: Electronic (PDF)
- Historical Range: 2018 - 2020
- Industry:Healthcare
Report Overview
The global drug device combination products market size was valued at USD 118.13 billion in 2021 and is expected to expand at a compound annual growth rate (CAGR) of 8.8% from 2022 to 2030. The market is primarily driven by the growing patient and physician preference for minimally invasive procedures and consistent-dosing treatment alternatives. The pandemic positively impacted this market space as the use of certain combination products increased during the different waves of COVID-19. In November 2020, the U.S. Food and Drug Administration has issued an emergency use authorization (EUA) for baricitinib in combination with remdesivir in hospitalized adults and pediatric patients two years of age and older who require supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation for suspected or laboratory-confirmed COVID-19 (ECMO).
第117条医疗器械监管(MDR)) requires manufacturers to obtain a Notified Body Opinion before placing drug-device combination goods on the market as an integral device and marketing them as a "medicinal product" (NBOp). The notified body then certifies that the device complies with the relevant General Safety and Performance Requirements (GSPR) and sends the manufacturer an NBOp Report to include in the Market Authorization Application (MAA). Autoinjectors, inhalers, pre-filled nebulizers, pre-filled pens, prefilled syringes, and transdermal patches are examples of drug-device combination items that require NBOp.
The subsequent increase in the adoption of these products can be credited to associated benefits such as reduced pain levels, better patient outcomes, reduced hospital stay, and overall healthcare cost-efficiency. Other advantages include synergistic effects facilitating multi-target treatment, improved tolerance levels, simplification of dosage regime, and improved symptomatic and pharmacokinetic profiles. These benefits are expected to propel the demand for these systems and present the market with numerous growth opportunities.
In addition, frequent intervention by government health organizations to ensure high patient safety is predominantly driving the market. To address the challenges associated with drug-device combination products as well as facilitate proper and consistent regulation, the U.S. FDA has established the Office of Combination Products (OCP). Moreover, unprecedented growth in the development of clinical drugs and devices by large pharmaceutical companies is believed to meet the demand for advanced medication delivery technologies. This has resulted in extensive product pipelines and maximized commercial returns on already established products, thereby serving as a significant driving factor for the industry.
Product Insights
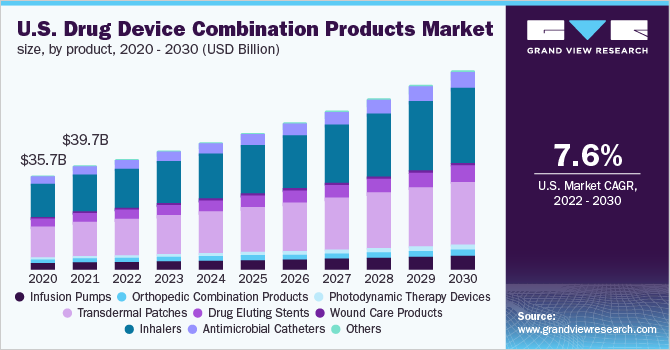
Transdermal patches emerged as the largest segment in 2021 with over 34.0% share. This can be attributed to the increasing demand for self-administration of drugs in cases of diseases requiring long-term treatment. For instance, in diabetes, insulin is needed to be frequently administered intravenously into the patient’s body. This repeated use of injections to deliver insulin causes pain and increases the risk of infection transmission, thus leading to heightened growth prospects for alternative drug delivery modes.
Advantages associated with the adoption of transdermal patches include better patient compliance and reduced risk of needlestick injuries and infections, which further accelerates the demand for these patches. Moreover, rising patient preference for pain-free drug delivery has led to a significant surge in the adoption of adhesive skin patches to administer drugs. This is expected to present the market with tremendous growth potential over the forecast period.
The inhalers segment is anticipated to exhibit a lucrative growth rate over the forecast period owing to the rising prevalence of chronic diseases such as tuberculosis, diabetes mellitus, and chronic obstructive pulmonary disease, which require more rigorous treatment. Technological advancements such as particle engineering, agglomerated vesicle technology, incorporation of MDI technology, and supercritical fluid technology are expected to boost segment growth.
Regional Insights
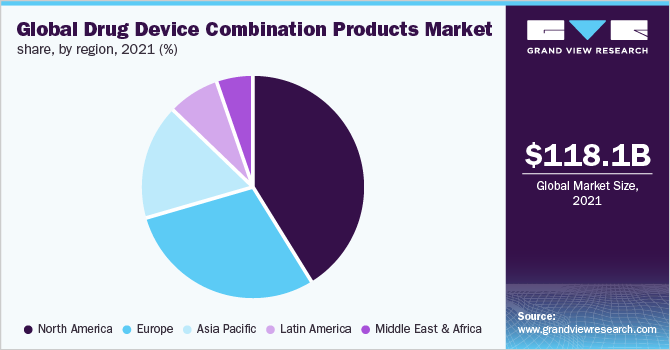
North America held the dominant share of over 40.0% in 2021. This can be attributed to the extensive new product development activities conducted by prominent players across this region. The rising chronic disease burden in this region is believed to be one of the key potential growth factors responsible for the high clinical urgency to incorporate these products. This is believed to contribute to the growth of the North American market.
Asia Pacific is anticipated to grow at a lucrative rate over the forecast period. The sizable growth is attributed to the increasing healthcare spending. Rising physician awareness levels pertaining to the benefits of these products, including low dosage requirement, timed release of medication, direct mitigation of device-centered infection using anti-infective medication in combination, and reduced systemic exposure, also contribute to the regional market growth. The market is driven by high R&D investments by global market players as well as increasing commercialization of these systems developed by key players.
Moreover, the urgent need for the development of new systems and replacement and upgrading of medical infrastructure is anticipated to present the APAC market with lucrative growth opportunities over the forecast period. Stringent regulatory policies issued by healthcare organizations to ensure high safety standards are further expected to widen the scope for growth across this region.
Key Companies & Market Share Insights
The key players are engaged in extensive expansion strategies, new product development activities, and mergers and acquisitions, which have significantly contributed to their growth. High operational costs, stringent regulatory framework, and capital requirements keep entry barriers at a higher level, owing to which, the threat of new entrants is expected to be low.
In 2016, Medtronic announced the U.S. launch of the MiniMed 630G System, an insulin pump. The product launch was carried out to provide diabetes mellitus treatment to patients aged 16 and above and facilitate personalized diabetes management. Earlier in 2016, the company introduced the New Resolute Onyx drug-eluting stent, commercially available in different sizes in Europe. This strategic initiative has supported Medtronic in expanding its geographical presence and developing treatment options for patients suffering from coronary artery disease. Some prominent players in the global drug device combination products market include
Abbott
Terumo Corporation
Stryker Corporation
Mylan N.V.
Medtronic
Allergan plc
Boston Scientific Corporation
Novartis AG
C.R. Bard
Teleflex Incorporated
W. L. Gore & Associates, Inc.
Drug Device Combination Products MarketReport Scope
Report Attribute |
Details |
Market size value in 2022 |
USD 127.81 billion |
Revenue forecast in 2030 |
USD 251.87 billion |
Growth Rate |
CAGR of 8.8% from 2022 to 2030 |
Base year for estimation |
2021 |
Historical data |
2018 - 2020 |
Forecast period |
2022 - 2030 |
Quantitative units |
Revenue in USD billion and CAGR from 2022 to 2030 |
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
Segments covered |
Product, region |
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
Country scope |
U.S.; Canada; U.K.; Germany; Japan; India; China; Brazil; Mexico; South Africa |
Key companies profiled |
Abbott; Terumo Corporation; Stryker Corporation; Mylan N.V.; Medtronic; Allergan plc; Boston Scientific Corporation; Novartis AG; C.R. Bard; Teleflex Incorporated; W. L. Gore & Associates, Inc. |
Customization scope |
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional, and segment scope |
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
Segments Covered in the Report
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends and opportunities in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global drug device combination products market report based on product and region:
Product Outlook (Revenue, USD Billion, 2018 - 2030)
Infusion Pumps
Volumetric
Disposables
Syringes
Ambulatory
Implantable
Insulin
Orthopedic Combination Products
Bone Graft Implants
Antibiotic Bone Cement
Photodynamic Therapy Devices
Transdermal Patches
Drug Eluting Stents
Coronary Stents
Peripheral Vascular Stents
Wound Care Products
Inhalers
Dry Powder
Nebulizers
Metered Dose
Antimicrobial Catheters
Urological
Cardiovascular
Others
Others
Regional Outlook (Revenue, USD Billion,2018 - 2030)
North America
U.S.
加拿大
Europe
Germany
U.K.
Asia Pacific
China
Japan
India
Latin America
Brazil
Mexico
MEA
South Africa
Frequently Asked Questions About This Report
b.The global drug device combination products market size was estimated at USD 118.1 billion in 2021 and is expected to reach USD 127.8 billion in 2022.
b.The global drug device combination products market is expected to grow at a compound annual growth rate of 8.8% from 2022 to 2030 to reach USD 251.8 billion by 2030.
b.North America dominated the drug device combination products market with a share of 41.35% in 2021. This is attributable to extensive new product development activities conducted by prominent players across this region.
b.Some key players operating in the drug device combination products market include Abbott; Terumo Corporation; Stryker Corporation; Mylan N.V.; Medtronic; Allergan plc; Boston Scientific Corporation; Novartis AG; C.R. Bard; Teleflex Incorporated; and W. L. Gore & Associates, Inc.
b.考虑的关键因素t are driving the drug device combination products market growth include growing patient and physician preference for minimally invasive procedures and consistent-dosing treatment alternatives.





