
Dermatology CRO Market Size, Share & Trends Analysis Report By Type (Preclinical, Clinical, Drug Discovery), By Service (Clinical Monitoring, Regulatory/Medical Affairs), By Region, And Segment Forecasts, 2021 - 2028
- Report ID: GVR-4-68039-635-9
- Number of Pages: 120
- Format: Electronic (PDF)
- Historical Range: 2016 - 2019
- Industry:Healthcare
Report Overview
The global dermatology CRO market size was valued at USD 4.14 billion in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 6.9% from 2021 to 2028. The demand for topical dermatological drugs such as anti-infective, anti-inflammatory, local anesthetics, emollients, and cleansers to treat acne is a primary factor driving the market. Furthermore, the dermatological contract research organization (CRO) business is being propelled forward by the growing awareness regarding skin disorders, the high need for rapid diagnosis, and the growth in the prevalence of skin cancer and other skin ailments. The COVID-19 epidemic hurt the economy in 2020 and continues to influence a variety of businesses around the world. Because of the introduction of virtual clinical trials and government measures to maintain broken healthcare supply chains, the market for dermatology CRO was mostly unscathed by the pandemic's ramifications. Furthermore, the bottleneck ofclinical trialsis improving as a result of continuous vaccination programs and the removal of shelter-in-place regulations. As a result, the market appears to have a bright future.
The rising cases of skin disorders across the globe are anticipated to augment the market growth over the forecast period. According to the American Academy of Dermatology Association, acne is the most common skin problem in the U.S., with up to 50 million people suffering from it each year. Atopic dermatitis affects one out of every ten persons at some point in their lives. Psoriasis affects around 7.5 million people in the U.S. Rosacea is a widespread skin condition that affects 16 million people in the U.S. Every day, more than 9,500 people in the U.S. are diagnosed with skin cancer.
The COVID-19 pandemic has highlighted the importance of virtual trials and the use of technology and software solutions. In the future years, the dermatology CRO environment is expected to be transformed by the increasing usage of machine learning-based platforms,artificial intelligence, and innovative trial designs. R&D activity is being driven by biopharmaceutical and pharmaceutical investments in novel and revolutionary therapies, such asregenerative medicine, and drug development services. Sponsors are still looking for experts and services in a variety of therapeutic areas.
Type Insights
临床部分占据了皮肤科反对tract research organizations market in 2020 with a revenue share of over 75.0%. The expanding number of biologics, the growing need for personalized medicines and orphan drugs, and the demand for innovative technologies are all contributing to the segment growth. The growth is expected to be fueled by factors such as technology advancements, globalization of clinical trials, and the need for CROs to conduct dermatology clinical trials.
Because phase III clinical trials are one of the most expensive stages of a clinical trial, outsourcing them to dermatology CROs helped the clinical segment to gain the largest revenue share in 2020. The preclinical segment is expected to expand at the highest CAGR of 8.5% over the forecast period. The rising number of preclinical trials involving large molecules and the growing need to reduce R&D costs are likely to drive the demand for dermatology CROs, resulting in market expansion.
Service Insights
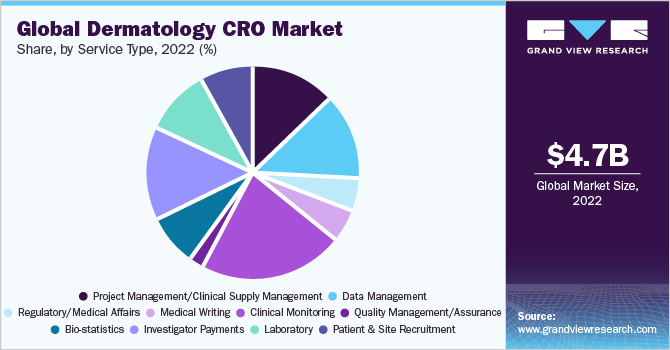
Clinical monitoring accounted for the largest revenue share of more than 20.0% in 2020 and is expected to maintain its position during the forecast period. This could be due to an increase in the number of clinical trials and the need to monitor those trials, which is driving up demand for services.
Over the last decade, dermatology clinical research has been outsourced to CROs for a variety of reasons, including cost-effectiveness and technical knowledge. The use of smart analytics and real-time data capture technologies in the healthcare sector is expected to improve clinical monitoring data. Real-time data collection on medication safety and toxicity allows for early detection of trial flaws and prompt corrections, such as trial re-design or termination, propelling the segment growth.
Phase Insights
In 2020, the phase III segment dominated the market with a revenue share of over 50.0% as phase III clinical studies are the costliest and involve a large number of participants. With 59 novel therapeutic medicines approved by the FDA between 2015 and 2016, the median cost of a single-phase III clinical study is roughly USD 19.0 million.
Phase III also necessitates a larger number of patients and, in many cases, a longer treatment term. The phase II segment held the second-largest revenue share in 2020. This research is split into two parts. The first step involves looking at a variety of doses as well as efficacy trials, and the second half involves deciding on a dose.
Regional Insights
In 2020, Asia Pacific dominated the market with a revenue share of over 40.0% and is expected to grow rapidly in the years to come. This is due to the high prevalence of chronic illnesses, the availability of different populations, the ease with which patients may be recruited and retained, and the implementation of laws that meet acceptance criteria. Furthermore, favorable government initiatives are aiding the market expansion. For example, the Central Drugs Standard Control Organization (CDSCO) issued new rules in February 2018 that are expected to cut the time it takes to get approval down to almost 30-60 days.
Due to the biggest number of trials conducted and outsourced in the area, the market for dermatology CROs in North America captured a significant revenue share in 2020. The growth of this regional market has also been fueled by the increasing government support for R&D activities through grants and money to research institutes and corporations. For example, the U.S. government spent USD 194.2 billion on medical and health research and development in 2018.
Key Companies & Market Share Insights
This market is becoming consolidated as various large CROs are undergoing inorganic growth. For instance, in February 2021, Icon plc entered into a definitive agreement to acquire PRA Health Sciences for around USD 12 billion. The combined business will be beneficial for customers owing to the increased geographic, functional, and therapeutic scale. Moreover, in 2021, Thermo Fisher underwent a definitive agreement to acquire PPD for USD 17.4 billion. This acquisition will help Thermo Fisher improve its presence significantly in the clinical research services industry, especially in the early phase clinical trials business.Healthcare contract research organizationsare working to offer their services on a global scale, in addition to improving their services. For example, Parexel increased its relationship with the Society for Clinical Research Sites (SCRS) in October 2019, the first and only global organization solely dedicated to protecting the interests of clinical research sites. As a Site Engagement Partner, the business launched several key initiatives aimed at improving the patient experience in clinical trials. Some prominent players in the global dermatology CRO market include:
IQVIA HOLDINGS INC.
Covance Inc.
Pharmaceutical Product Development, LLC (PPD)
Parexel International Corporation
Charles River Laboratories International, Inc.
Icon, Plc
Medidata Solutions, Inc.
Syneos Health
Pharmaron
GVK Biosciences Private Limited
Wuxi AppTec
MEDPACE HOLDINGS, INC.
PRA Health Sciences
CTI Clinical Trial & Consulting
Bioskin
Proinnovera
Biorasi
Javara
TFS
Dermatology CRO Market Report Scope
Report Attribute |
Details |
Market Size value in 2021 |
USD 4.43 billion |
Revenue forecast in 2028 |
USD 7.04 billion |
Growth rate |
CAGR of 6.9% from 2021 to 2028 |
Base year for estimation |
2020 |
Historical data |
2016 - 2019 |
Forecast period |
2021 - 2028 |
Quantitative units |
Revenue in USD million/billion and CAGR from 2021 to 2028 |
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
Segments covered |
Type, service, region |
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
Country scope |
U.S.; Canada; U.K.; Germany; France; Italy; Spain; India; Japan; China; Australia; South Korea; Brazil; Mexico; Argentina; Colombia; Chile; South Africa; Saudi Arabia; UAE; Iran; Israel |
Key companies profiled |
IQVIA; ICON Plc; Charles River Laboratory; Pharmaceutical Product Development, LLC |
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
革命制度党cing and purchase options |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
Segments Covered in the Report
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends and opportunities in each of the sub-segments from 2016 to 2028. For the purpose of this study, Grand View Research has segmented the global dermatology CRO market report on the basis of type, service, and region:
Type Outlook (Revenue, USD Million, 2016 - 2028)
Drug Discovery
Target Validation
Lead Identification
Lead optimization
Preclinical
Clinical
Phase I
Phase II
Phase III
Phase IV
Service Outlook (Revenue, USD Million, 2016 - 2028)
Project Management/Clinical Supply Management
Data Management
Regulatory/Medical Affairs
Medical Writing
Clinical Monitoring
Quality Management/Assurance
Bio-statistics
Investigator Payments
Laboratory
Patient and Site Recruitment
Technology
Others
Regional Outlook (Revenue, USD Million, 2016 - 2028)
North America
U.S.
Canada
Europe
Germany
U.K.
France
Italy
Spain
Asia Pacific
China
Japan
India
Australia
South Korea
Latin America
Brazil
Mexico
Argentina
Colombia
Chile
Middle East & Africa
South Africa
Saudi Arabia
UAE
Iran
Israel
Frequently Asked Questions About This Report
b.The global dermatology CRO market size was estimated at USD 4.14 billion in 2020 and is expected to reach USD 4.43 billion in 2021.
b.The global dermatology CRO market is expected to grow at a compound annual growth rate of 6.9% from 2021 to 2028 to reach USD 7.04 billion by 2028.
b.The Asia Pacific dominated the dermatology contract research organization market, accounting for 42.0% in 2020. This is due to the high prevalence of chronic illnesses, the availability of different populations, the ease with which patients may be recruited and retained, and the implementation of laws that meet acceptance criteria.
b.Some key players operating in the dermatology contract research organization market include IQVIA HOLDINGS INC., Covance Inc., Pharmaceutical Product Development, LLC (PPD), Parexel International Corporation, Charles River Laboratories International, Inc., Icon, Plc, Medidata Solutions, Inc., Syneos Health, Pharmaron, GVK Biosciences Private Limited, Wuxi AppTec, MEDPACE HOLDINGS, INC., PRA Health Sciences, CTI Clinical Trial & Consulting, Bioskin, Proinnovera, Biorasi, Javara, and TFS.
b.The dermatological CRO business is being propelled forward by growing awareness of skin disorders, a high need for rapid diagnosis, and growth in the prevalence of skin cancer and other skin ailments.





