
Congestive Heart Failure Treatment Devices Market Size, Share & Trends Analysis Report By Product (Ventricular Assist Devices, Counter Pulsation Devices, Implantable Cardioverter Defibrillators, Pacemakers) And Segment Forecasts to 2016 - 2024
- Report ID: GVR-1-68038-292-1
- Number of Pages: 105
- Format: Electronic (PDF)
- Historical Range: 2013 - 2014
- Industry:Healthcare
Report Overview
The Congestive Heart Failure (CHF) treatment devices market was valued at USD 15.6 billion in 2015 and is expected to witness a CAGR of 8.1% over the forecast period. The rising burden of Cardiovascular Diseases (CVDs) is one of the prime factors responsible for the lucrative growth of the CHF treatment devices market. Sedentary lifestyles, mental stress, and junk food consumption are other key factors associated with the development of CVDs.
Global congestive heart failure treatment devices market, by product
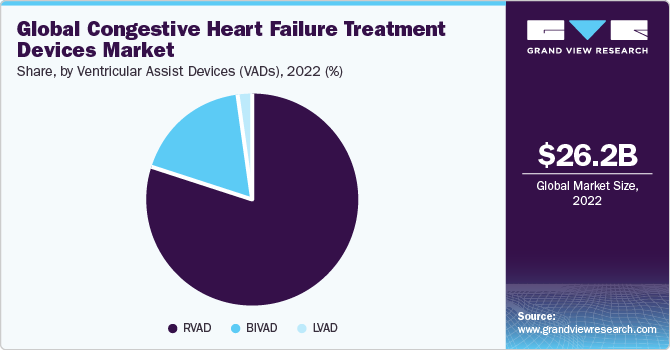
CVDs are a group of disorders characterized by functional or structural abnormalities or both that affect the functioning of the heart and the associated blood vessels. According to the World Health Organization, CVDs contribute to the maximum number of deaths globally. In 2012, approximately 17.5 million deaths were reported from CVDs, representing 31.0% of the global death statistics. Of the 17.5 million deaths reported, 7.4 million were due to coronary heart disease and 6.7 million were due to stroke.
Favorable reimbursement policies, such as the U.S. Medicare system, is a prime growth factor for this vertical. Medical device companies now provide new product platforms, which are covered under reimbursement programs that benefit the patient. The reimbursement can be availed for devices, such as VADs, ICDs, and pacemakers that are used to treat CHF.
Individuals above 60 years of age forms the key target population for CHF treatment devices. This demographic is characterized with low immunity levels and are prone to chronic diseases including CVDs; and thereby serve as a high impact rendering driver for the growth of these products over the forecast period. As a result, the latest treatments available in cardiac care are expected to increase the life span of the elderly population subset. The chart below forecasts the number of geriatric patients over the next six years, which form the routine target group contributing to the rise in surgical volumes.
Product Insights
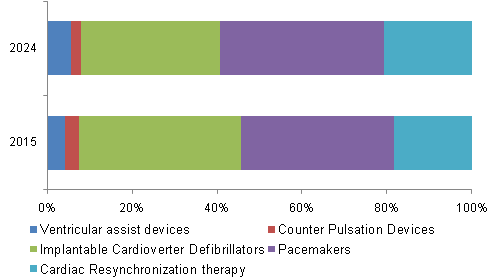
The Implantable Cardioverter Defibrillators (ICDs) held a lucrative share in the market due to its ability to perform three basic functions, which include defibrillation, cardioversion, and pacing of the heart. The ICD segment consisting of two types, transvenous and subcutaneous, held a lucrative share in 2015, which was followed by the pacemaker’s segment. This can be attributed to the fact thatdefibrillatorsand心脏起搏器form the primary line of treatment administered for heart failure. On the other hand, the VAD segment is anticipated to grow at a significant rate over the forecast period due to reimbursement coverage and technological advancements in VADs
North America congestive heart failure treatment devices market, by product
North America dominated the overall industry in terms of revenue share at over 40.0% in 2015; this can be attributed to the presence of a large population base suffering from cardiovascular disorders coupled with a significant geriatric population base. Asia-Pacific is expected to grow at the fastest CAGR during the forecast period and its share is expected to grow, owing to the increasing demand for these products. Furthermore, the rapidly improving healthcare infrastructure in the countries, such as India and Japan, also contribute toward the growth of this region.
据美国心脏Association, 17.3 million deaths occur due to cardiovascular disorders, and this number is expected to rise to 23.6 million by 2030. This high prevalence resulted in greater usage of this equipment in this region. In addition, developed healthcare infrastructure in the U.S. facilitated access to advanced therapeutics. This contributed to the growth of this region. Moreover, the availability of reimbursement for cardiovascular therapies supported the growth substantially.
Congestive Heart Failure Treatment DevicesMarket Share
Some of the market players in this vertical include Medtronic Plc, Boston Scientific Corporation, Biotronik SE & Co., KG, St. Jude Medical, among others. The operating strategies adopted by these players include new product launches and regional expansions. For instance, in November 2015, Biotronik launched Biomonitor 2 in Europe, which is an implantable cardiac remote monitor. The product launch is anticipated to help the company expand its existing product portfolio in Europe.
Congestive heart failure treatment devices market: Competitive Scenario
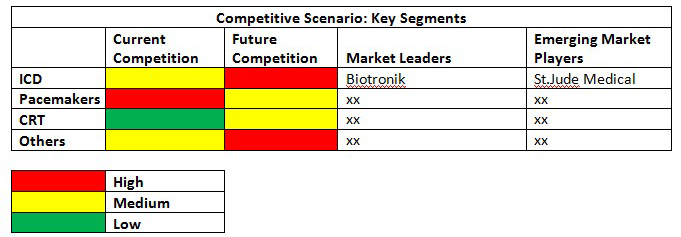
Biotronik also launched Ilesto series of the CRT-D system in May 2013, post-FDA-approval for the treatment of atrial fibrillation. The product launch will aid Biotronik in expanding its cardiac rhythm management product offerings.
Congestive Heart Failure Treatment Devices Market Report Scope
Report Attribute |
Details |
Market size value in 2020 |
USD 23.5 billion |
Revenue forecast in 2024 |
USD 32.0 billion |
Growth Rate |
CAGR of 8.1% from 2016 to 2024 |
Base year for estimation |
2015 |
Historical data |
2013 - 2014 |
Forecast period |
2016 - 2024 |
Quantitative units |
Revenue in USD million and CAGR from 2016 to 2024 |
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
Segments covered |
Product, region |
Regional scope |
北美;欧洲;亚太地区;拉丁美洲; Middle East & Africa |
Country scope |
U.S.; Canada; Germany; U.K.; Japan; China; Brazil; Mexico; South Africa; Saudi Arabia |
Key companies profiled |
Medtronic Plc; Boston Scientific Corporation; Biotronik SE & Co., KG; St. Jude Medical |
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
Market Segments Covered in the Report
This report forecasts revenue growth and provides an analysis of the market trends in each of the sub-markets from 2013 to 2024. For the purpose of this study, Grand View Research has segmented remote patient monitoring devices market on the basis of product and region:
- Global CHF Treatment Devices Market by Product(百万美元),2013年- 2024
Ventricular Assist Devices (VADs)
LVAD
RVAD
BiVAD
Counter Pulsation Devices
Implantable Cardioverter Defibrillators
Transvenous ICD
Subcutaneous ICD
Pacemakers
Implantable
External
Cardiac Resynchronization therapy
Cardiac Resynchronization Therapy-Defibrillators (CRT-D)
Cardiac Resynchronization Therapy-Pacemakers (CRT-P)
- CHF Treatment Devices Market by Region (百万美元),2013年- 2024
North America
The U.S.
Canada
Europe
The U.K.
Germany
Asia Pacific
Japan
China
拉丁美洲
Brazil
Mexico
MEA
South Africa
Saudi Arabia
Frequently Asked Questions About This Report
b.The global congestive heart failure treatment devices market size was estimated at USD 21.9 billion in 2019 and is expected to reach USD 23.5 billion in 2020.
b.The global congestive heart failure treatment devices market is expected to grow at a compound annual growth rate of 8.1% from 2016 to 2024 to reach USD 32.0 billion by 2024.
b.Pacemakers dominated the congestive heart failure treatment devices market with a share of 37.8% in 2019. This can be attributed to the fact that pacemakers form the primary line of treatment administered for heart failure.
b.Some key players operating in the congestive heart failure treatment devices market include Medtronic Plc; Boston Scientific Corporation; Biotronik SE & Co., KG; and St. Jude Medical
b.Key factors that are driving the market growth include rising burden of Cardiovascular Diseases (CVDs), sedentary lifestyles, mental stress, junk food consumption, and favorable reimbursement policies.





