
CAR T-cell Therapy Market Size, Share & Trends Analysis Report by Product (Abecma, Breyanzi), By Disease Indication (Lymphoma, Leukemia, Multiple Myeloma), By End-use, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68040-090-4
- Number of Pages: 150
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry:Healthcare
Report Overview
The globalCAR T-cell therapy market sizewas valued atUSD 2.75 billion in 2022and is expected to grow at a compound annual growth rate (CAGR) of 23.32% from 2023 to 2030. Remarkable success in treating blood cancers, such asacute lymphoblastic leukemia(ALL) and non-Hodgkin lymphoma (NHL) has increased the adoption of CAR T-cell therapy and is anticipated to boost the industry's growth. CAR T-cell therapy provides a highly targeted treatment that specifically targets cancer cells and leaves the healthy cell unharmed. Such advantages are projected to further fuel growth. The COVID-19 pandemic resulted in a negative impact on the usage and manufacturing of CAR T-cells. It was owing to the management of the therapy and the extensive time required throughout the process, requiring specialized personnel and coordinated work systems, which were hampered during the pandemic.
Additionally, the closing of borders and reduction of transit of people affected the use of CAR T-cell Therapy. Lastly, throughout 2020, access to life-saving drugs was negatively impacted, which curated negative impacts for CAR T-cell therapy recipients. Lastly, the COVID-19 pandemic disrupted manyclinical trials, including those for CAR T-cell therapy. This resulted in a reduced number of clinical trial participants and delays in the development of new treatments.
CAR T-cell Therapy represents a strong paradigm treatment shift in the overall cancer treatment approach. The CAR T-cell approach utilizes genetically modified cytotoxic immune T-cells to approach tumor-specific antigens, which helps in durable remissions of relapsed or refractory B-Cell Lymphoma. B-Cell lymphoma is the most common type of malignant lymphoma, and relapsed or refractory lymphoma has shown a higher cause of treatment failure. For instance, as per Cancer Network 2022, diffuse large B-cell lymphoma (DLBCL) holds a significant presence under the category of Non-Hodgkin Lymphoma and approximately 30-40% of the patients develop relapsed/refractory DLBCL during the first 2 years. Thereby, the introduction of CAR T-cell therapy has shown immense promise for cancer treatment, and the side effects of the therapy can be properly managed.
癌症恶性肿瘤的生长情况下继续to propel the market for CAR T-cell therapy. Additionally, various product launches and approvals provide significant traction to the market players. CAR T-cell Therapy is immunotherapy using genetically modified T cells to attack cancer cells in the body. The process involves using the patient’s T cells and engineering them to express chimeric antigen receptors (CAR) on the surface. The following CARs are specifically designed to recognize and bind to specific proteins in cancer cells, which in turn triggers the T cells to kill the cancer cells.
Product launches and timely approvals have led to strategic competencies in the utilization of CAR T-cell therapy. For instance, in May 2023, Bristol Myers Squibb received approval for its CD19-directed CAR T-cell therapy, Breyanzi, from the European Commission. The therapy has application in the treatment of diffuse large B-cell lymphoma in adult patients. Similarly, in February 2022, Cltacabtagene autoleucel (Carvykti) for adults suffering from multiple myeloma was approved by the U.S. FDA. The following approval is second in line and provides an alternative for people suffering from multiple myeloma via CAR T-cell therapy.
Product Insights
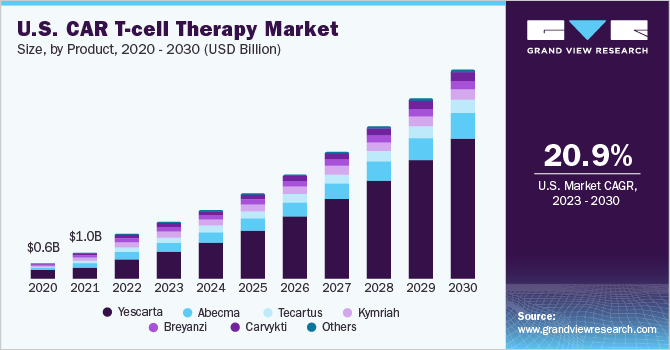
Yescarta segment held the major share of 43.76% of the CAR T-cell therapy market in 2022. It is also expected to be the fastest-growing product segment during the forecast period. Yescarta is also known as axicabtagene ciloleucel and targets the CD19 antigen. Yescarta is primarily prescribed for large B-cell lymphoma when the first treatment does not work, or cancer returns within the first year of treatment, or when at least two kinds of treatment for follicular lymphoma have failed. The strong share can be attributed to the improved survival rates for adults with relapsed large B-cell lymphoma. For instance, as per Gilead 2022, around 2 times more patients taking Yescarta (40.5%) were alive for two years without disease progression, and the median event-free survival was four-fold greater than the current standard of care.
The Carvykti segment is expected to witness lucrative growth in the CAR T-cell therapy market by 2030. It is expected to grow at a CAGR of 15.68%在2023 - 2030。我Carvykti也是一个自体mmunotherapy to find and destroy B cell maturation antigen or BCMA-expressing cells. Strong regulatory support in the forms of clinical trials and approvals has allowed the cancer drug to grow exponentially. For instance, on February 28, 2022, Ciltacabtagene autoleucel was approved by the U.S. FDA, promising a wider application, specifically for patients suffering from multiple myeloma, relapsed or refractory. Moreover, clinical trials have shown robust results with around 98% of the study participants responding to the treatment, with 78% of participants showing no signs of cancer in bone marrow or blood. The results lasted for a median of 22 months showing a strong premise for treatment in cancer patients.
Disease Indication Insights
The Lymphoma segment held the largest share of 57.15% of the CAR T-cell therapy market in 2022. It is also expected to be the fastest-growing application segment. The dominant market share can be attributed to the presence of various CAR-T drugs focusing to target lymphoma. For example, Breyanzi, Kymirah, Tecartus, and Yescarta help in targeting the CD-19 antigen which in turn helps in eliminating large B-cell lymphoma in both adults and children. Additionally, lymphoma has a significant prevalence across the world which allows CAR T-cell therapy to eliminate and mitigate life-threatening conditions. As per Globocon 2020, nearly 544,000 new cases of non-Hodgkin lymphoma were observed in the world, with nearly 259,000 deaths recorded in the same year.
Multiple Myeloma is expected to be the second fastest-growing segment in the CAR T-cell therapy market by 2030. It is expected to grow at a CAGR of 22.05% between 2023-2030. The global incidence of multiple myeloma has been on the increase. As per a report published by the Lancet Hematology 2020, the age-standardized rate of multiple myeloma was 1.78 per 100,000 people globally. The research highlighted a steep increase in the overall incidence from countries where men aged 50 or older are relatively high. The U.S. FDA continues to support product approvals for CAR T therapy drugs which will help in reducing the mortality of multiple myeloma cases. For instance, in April 2021, the idecabtagene vicleucel (Abecma) was approved by the U.S. FDA for people with multiple myeloma, in tandem with those who have not responded to or wherein, the condition has resurfaced post four different cancer treatments.
End-use Insights
The hospital segment held the largest share of 56.26% of the CAR T-cell therapy industry in 2022. The dominant market share can be attributed to the presence of robust technology, well-equipped operating facilities like theatres, and strong buying power for hospitals across major geographies. Furthermore, hospitals that leverage the in-house CAR T process can reduce the overall cost pertinent to the therapy, which increases the footprint towards such spaces. For instance, Sheba Hospital in Israel utilizes in-house processes pertinent to the process which reduces the overall treatment time to less than 10 days and exponentially reduces costs by at least 50%. As per the company website, nearly 250 patients have undergone CAR T therapy in the hospital since 2015, and have amassed patients from across the world.
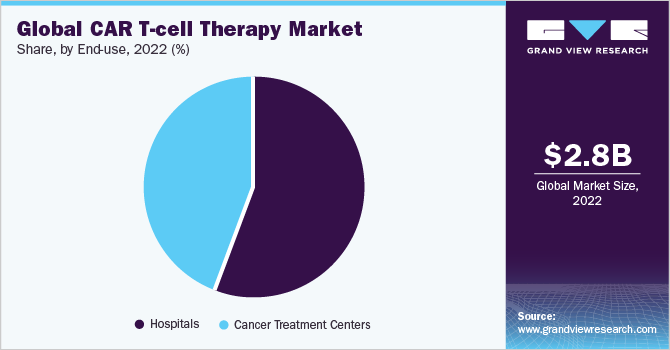
Cancer Treatment Centers are expected to be the fastest-growing segment in the CAR T-cell therapy industry by 2030. It is expected to grow at a CAGR of 24.27%between 2023-2030. It is owing to the availability of a wide range of treatment choices and patient comfort options. Additionally, patients are treated within the communities which increases the overall CAR T Treatment reach. Lastly, strategic initiatives taken by renowned administrative institutions will enhance patient coverage. For instance, in February 2023, UVM Cancer Center in Vermont U.S. announced the availability of new CAR T-cell treatment for patients suffering from blood cancer.
Regional Insights
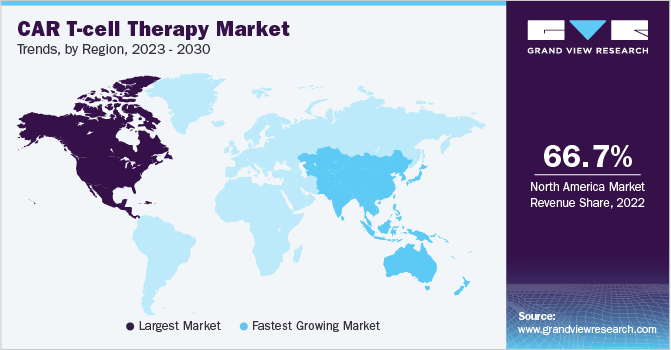
North America accounted for the largest share of the CAR T-cell therapy industry in 2022 and accounted for around 66.70%of the total market revenues. The presence of major companies, increased government funding, and research activities, along with strong coverage via various insurance policies allow the region to hold a strong position. The cost pertinent to CAR T therapies on average reaches $400,000 and above, thereby, strong insurance policies guarantee patient comfort and market proliferation.
For instance, in August 2019, the U.S. Centers for Medicare and Medicaid Services announced coverage for CAR T-cell therapies. The move allows for covering a definitive spectrum of the senior population in the region, in which blood cancer discoveries are high. As per the Federation of American Scientists 2023, nearly 60 million individuals, or 18.2% of the entire U.S. population are covered under Medicare.
Asia-Pacific is estimated to be the fastest-growing region in the CAR T-cell therapy industry by 2030. It is expected to grow at a CAGR of 27.64%between 2023-2030. The fast growth is due to the growing patient pool in the region along with statutory support for the products and market players. Moreover, increasing efforts by major players in the market for the development of technologically advanced therapies are also contributing to the market growth. For instance, as per Clinical Trials Database 2023, around 103 CAR T Therapies are going through active clinical phases in China while nearly 5 studies are in the pipeline in Australia for CAR T Therapy.
Key Companies & Market Share Insights
The high demand for CAR T-cell therapy for mitigating relapsed or refracted blood cancer presents numerous market opportunities for major players to capitalize on. Major players are adopting various strategies such as acquisitions, partnerships, collaborations, expansions, and mergers to expand their market share and strengthen their market position. For instance, in April 2023, Wisconsin Alumni Research Foundation (WARF) and Ginkgo Bioworks entered a partnership to develop next-generation GD2 CAR T-cell Therapies. Some prominent players in the global CAR T-cell therapies market include:
Bristol-Myers Squibb Company
Novartis AG
Gilead Sciences, Inc.
Johnson & Johnson Services, Inc.
JW Therapeutics (Shanghai) Co., Ltd.
bluebird bio, Inc.
Merck & Co., Inc.
Sangamo Therapeutics
Sorrento Therapeutics, Inc.
GSK plc.
CAR T-Cell Therapy Industry Report Scope
Report Attribute |
Details |
Market size value in 2023 |
USD 3.68 billion |
Revenue forecast in 2030 |
USD 15.97 billion |
Growth rate |
CAGR of 23.32% from 2023 to 2030 |
Base year for estimation |
2022 |
Historical data |
2018 - 2021 |
Forecast period |
2023 - 2030 |
Quantitative units |
Revenue in USD million, CAGR from 2023 to 2030 |
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, trends |
赛格ments covered |
Product, disease indication, end-use, region |
Regional scope |
North America; Europe; Asia Pacific; Rest of the World |
有限公司untry scope |
U.S., Canada, UK, Germany, Japan, China |
Key companies profiled |
Bristol-Myers Squibb Company; Novartis AG; Gilead Sciences, Inc.; Johnson & Johnson Services, Inc.; JW Therapeutics (Shanghai) Co., Ltd.; Bluebird Bio, Inc.; Merck & Co., Inc; Sangamo Therapeutics; Sorrento Therapeutics, Inc.; GSK plc. |
Customization scope |
Free report customization (equivalent up to 8 analysts’ working days) with purchase. Addition or alteration to country, regional & segment scope. |
Global CAR T-cell Therapy Market Report Segmentation
This report forecasts revenue growth at global, regional, & country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this report, Grand View Research has segmented global CAR T-cell Therapy industry report based on product, disease indication, end-use, and region:
Product Outlook (Revenue, USD Million, 2018 - 2030)
Abecma (idecabtagene vicleucel)
Breyanzi (lisocabtagene maraleucel)
Carvykti (ciltacabtagene autoleucel)
Kymriah (tisagenlecleucel)
Tecartus (brexucabtagene autoleucel)
Yescarta (axicabtagene ciloleucel)
Others
Disease Indication Outlook (Revenue, USD Million, 2018 - 2030)
Leukemia
Lymphoma
Multiple Myeloma
Others
End-Use Outlook (Revenue, USD Million, 2018 - 2030)
Hospitals
Cancer Treatment Centers
Regional Scope Outlook (Revenue, USD Million, 2018 - 2030)
North America
U.S.
Canada
Europe
UK
Germany
Rest of Europe
Asia Pacific
Japan
China
Rest of Asia Pacific
Rest of World
Frequently Asked Questions About This Report
b.The global CAR T-cell therapy market size was valued at USD 2.75 billion in 2022 and is expected to reach USD 3.68 billion in 2023.
b.The global CAR T-cell therapy market is expected to grow at a compound annual growth rate of 23.32% from 2023 to 2030 to reach USD 15.97 billion by 2030.
b.Lymphoma segment held the major share of 57.15% of the CAR T-cell therapy market in 2022. It is also expected to be the fastest growing application segment. The dominant market share can be attributed to the presence of various CAR T drugs focusing to target lymphoma.
b.Some of the key players in the global CAR T-cell therapies market include Bristol-Myers Squibb Company, Novartis AG, Gilead Sciences, Inc., Johnson & Johnson Services, Inc., and JW Therapeutics (Shanghai) Co., Ltd.
b.The rising prevalence of cancer such as multiple myeloma across the world and the rampant regulatory support in the form of product approvals and an intensive product pipeline of drugs are some of the key factors driving the growth of the market.





