
Canada Varicose Vein Treatment Devices Market Size, Share & Trends Analysis Report By Type (Endovenous Ablation, Sclerotherapy), By End Use (Ambulatory Care Unit, Hospitals, Vein Clinics), By Region, And Segment Forecasts, 2021 - 2028
- Report ID: GVR-4-68038-326-3
- Number of Pages: 74
- 格式:选择ronic (PDF)
- Historical Range: 2016 - 2019
- Industry:Healthcare
Report Overview
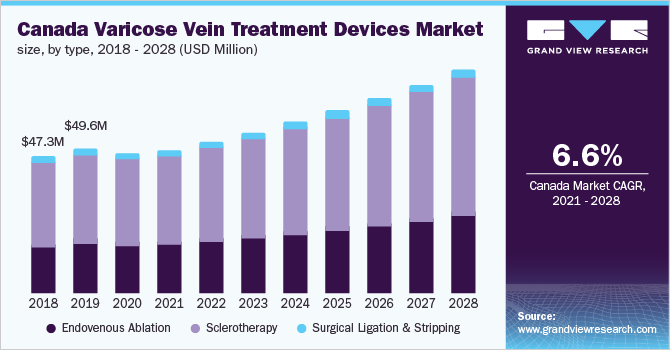
The Canada varicose vein treatment devices market size was valued at USD 48.0 million in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 6.6% over the forecast period. The rising cases of varicose veins and increasing adoption of endovenous ablation are key factors driving the growth of the market. According to a study published in National Center for Biotechnology Information (NCBI), nearly 3.7 million people have varicose veins in Canada. Furthermore, rising awareness among patients regarding venous diseases is anticipated to favor market growth. The rising demand for minimally invasive treatment options, approval from Health Canada for various ablation devices & sclerotherapies, and positive recommendations from associations, such as Ontario Health Technology Advisory Committee (OHTAC) for Endovenous Laser Ablation (EVLA) & Radiofrequency Ablation (RFA), is anticipated to drive the market.
The non-invasive techniques for treating varicose veins have been found to have similar effectiveness as invasive surgeries as per the latest studies, resulting in more preference for minimally invasive procedures. These minimally invasive procedures have a lot of other merits over invasive surgeries. In recent times, technological advancements have resulted in the emergence of minimally invasive surgeries. Endovenous ablation & sclerotherapy are noninvasive surgeries for treating varicose veins. Endovenous ablation consists of radiofrequency and laser procedures. The radiofrequency procedure is used more where the vein is thermally heated at a temperature of 60 °C to 100 °C. Sclerotherapy involves injecting a salt solution into the vein irritating the blood vessel lining, collapsing & sticking together making a blood clot. These minimally invasive techniques are being preferred more in recent years over invasive surgeries.
COVID-19 Canada Varicose Vein Treatment Market Impact: 3.2% decline from 2019 to 2020
Pandemic Impact |
Post-COVID Outlook |
According to the Canadian Institute for Health Information, the number of varicose vein surgeries/vein repairs from March to June 2020 witnessed a double-digit decline compared to 2019 |
Vaccination, lockdown upliftment, and relaxations in social restrictions are expected to allow hospitals & clinics to resume the treatment for varicose veins with preventive measures & guidelines. Many vein clinics have started offering virtual consultations to patients. This is expected to have a positive impact on market growth in the coming year. |
In response to the COVID-19 pandemic and declining revenue, key manufacturers implemented cost reduction strategies, such as a decrease in meetings, travels, and customer events, hiring, and certain clinical research & development programs |
As clinics and hospitals are resuming services for non-emergent cases across the nation, backlogged procedures for varicose veins are anticipated to drive the demand for devices |
In Canada, reimbursement and health insurance coverage for varicose veins depends on the type of treatment. Public healthcare in Canada provides coverage for conventional vein stripping and ligation surgery & sclerotherapy. The Ontario Health Insurance Plan (OHIP) emphasizes conservative management of varicose veins before more invasive procedures. In 2019, the Ontario Health Technology Advisory Committee (OHTAC) recommended coverage for compression therapy for patients; however, medical-grade compression stockings are not publicly funded for most patients.
Type Insights
Based on type, the market is categorized into endovenous ablation, sclerotherapy, and surgical ligation & stripping. The sclerotherapy segment accounted for the largest revenue share of over 62% in 2020 on account of high demand due to easier accessibility and efficiency to treat varicose veins. Endovenous ablation & sclerotherapy are less costly compared to vein surgery. The hospital stay is longer with several side effects of invasive open vein surgery. In addition, recurrence after 4 to 5 years is observed. Minimally invasive procedures do not need hospitalization as they are daycare procedures with fewer side effects and negligible recurrence.
These factors are resulting in a gradual shift toward minimally invasive procedures, which is likely to significantly boost the segment growth. The endovenous ablation segment is further categorized into endovenous laser ablation and radiofrequency ablation. The growth of the segment can be attributed to the rapid adoption of laser therapy for the treatment of varicose veins. Numerous market players are investing in the development of innovative and effective products. Commercialization of various products in recent years is anticipated to further boost market growth. For instance, in February 2020, biolitec AG announced the launch of ELVeS Radial 2ring Pro, an advanced laser fiber for endovenous laser treatment of severely tortuous varicose veins.
End-use Insights
基于最终用途,市场划分为ambulatory care units, vein clinics, and hospitals. The hospital's segment accounted for the largest share of over 51% in 2020 owing to the high number of public and private hospitals in Canada. Moreover, expensive equipment, such as an endovenous ablation system, can be easily purchased by hospitals. This factor along with favorable health policies for open vein surgery is fueling the growth of the hospital's segment.
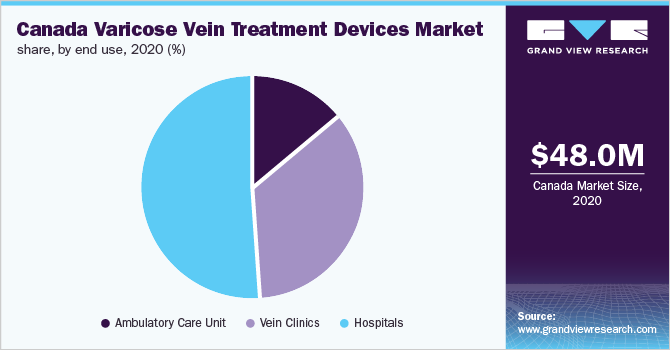
The vein clinics segment accounted for the second-largest market share and is expected to grow at the fastest CAGR from 2021 to 2028. An increasing number of cosmetic clinics offering minimally invasive treatments for varicose veins is driving the vein clinics segment. The rising number of vascular surgeons or physicians with standalone vein clinics is also expected to drive the growth of the segment.
Moreover, many vein clinics, such as the Canadian Vein Institute, Canada Vein Clinics, and The Vein Treatment Centre, offer endovenous ablation treatment, which is preferred by patients. As a result, the popularity of vein clinics is gradually increasing, thereby supporting the segment growth.
Key Companies & Market Share Insights
Acquisitions, partnerships, and the launch of new products are key strategies adopted by market players. For instance, in August 2019, Boston Scientific Corp. acquired BTG, which resulted in the inclusion of Varithena and the expansion of its portfolio in minimally invasive surgical devices. In December 2018, Merit Medical Systems acquired the assets of Vascular Insights, LLC, which resulted in the inclusion of ClariVein in the company’s product portfolio. Some of the prominent players in the Canada varicose vein treatment devices market include:
Angiodynamics
Medtronic
Teleflex Incorporated
Sciton, Inc.
Biolitec AG
Dornier Medtech
Merit Medical Systems
阿尔玛激光
Boston Scientific Corp.
Canada Varicose Vein Treatment Devices MarketReport Scope
Report Attribute |
Details |
Market size value in 2021 |
USD 49.3 million |
Revenue forecast in 2028 |
USD 77.0 million |
Growth rate |
CAGR of 6.6% from 2021 to 2028 |
Base year for estimation |
2020 |
Historical data |
2016 - 2019 |
Forecast period |
2021 - 2028 |
Quantitative units |
Revenue in USD million/billion and CAGR from 2021 to 2028 |
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
Segments covered |
Type and end use |
Country scope |
Canada |
Key companies profiled |
Angiodynamics; Medtronic; Teleflex Incorporated; Sciton, Inc.; Biolitec AG; Dornier Medtech; Merit Medical Systems; Alma Lasers; Boston Scientific Corp. |
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
Segments Covered in the Report
This report forecasts revenue growth at a country level and provides an analysis of the latest industry trends in each of the sub-segments from 2016 to 2028. For the purpose of this study, Grand View Research has segmented the Canada varicose vein treatment devices market report on the basis of type and end use:
Type Outlook (Revenue, USD Million, 2016 - 2028)
Endovenous Ablation
Endovenous Laser Ablation (EVLA)
Fetal Radiofrequency Ablation (RFA)
Sclerotherapy
Surgical Ligation & Stripping
End-use Outlook (Revenue, USD Million, 2016 - 2028)
Ambulatory Care Unit
Vein Clinics
Hospitals
Frequently Asked Questions About This Report
b.The Canada varicose veins treatment devices market size was estimated at USD 48.0 million and is expected to reach USD 49.3 million in 2021.
b.The Canada varicose veins treatment devices market is expected to grow at a compound annual growth rate of 6.58% from 2021 to 2028 to reach USD 77.0 million by 2028.
b.Sclerotherapy dominated the Canada varicose veins treatment devices market with a share of 62% in 2020. This is attributable to the favorable public & private health reimbursement and shorter duration of the surgery & recovery period.
b.Some key players operating in the Canada varicose veins treatment devices market include Medtronic; Boson Scientific Corporation; Alma Lasers; Merit Medical Systems; Sciton, Inc.; AngioDynamics; Teleflex Incorporated; biolitec AG; and Dornier Medtech.
b.Key factors that are driving the market growth include the rising prevalence of varicose veins, increasing adoption of endovenous ablation, and rising awareness among patients regarding venous diseases.





