
先进的治疗药品CDMO市场Size, Share & Trends Analysis Report By Product (Gene Therapy, Cell Therapy, Tissue Engineered), By Phase, By Indication, By Region, And Segment Forecasts, 2021 - 2028
- Report ID: GVR-4-68039-676-6
- Number of Pages: 100
- Format: Electronic (PDF)
- Historical Range: 2016 - 2019
- Industry:Healthcare
Report Overview
The global advanced therapy medicinal products CDMO market size was valued at USD 5.29 billion in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 12.0% from 2021 to 2028. The growing demand for advanced therapy is the key factor fueling the market growth. The growth is attributed to the increasing prevalence of rare and life-threatening diseases, such as metabolic and optical diseases, and rising investment in R&D of advanced therapy medicinal products. Besides, ATMPs such as mesenchymal stem cells (MSCs) are a new treatment effective against the COVID-19 virus.
The COVID-19 pandemic has had a massive impact on the development and manufacturing of advanced therapy medicinal products. The number of clinical trials for ATMPs increases due to the efficacy of the product such as MSCs treatment to fight against the virus. The FDA has established a special program for the acceleration of possible therapies for COVID-19 treatment. The program extends support for continuous activity in the clinical trials space and testing of drug molecules for the virus, which will ensure the safety and effectiveness of the drug. Gene andcell therapyhave been identified as a potential treatment for the COVID-19 virus, as a result, the regulatory authorities have accelerated the drug development process and supply chain management.
In the last few years, the development of advanced therapy medicinal products has been growing as the demand for the product rises. In 2020, there were around 1,078 ongoing clinical trials of ATMPs globally. With the rising demand for the products and tremendous growth of gene and cell therapy, the role of CDMO has also increased. The biopharmaceutical companies outsource the manufacturing services to CDMOs to focus on the core functions of the organization. CDMO provides a complete package right from the planning for the clinical trial to the actual manufacturing of the drugs. They also help distribute the high cost of research for these products over a period, based on the contracts.
Many organizations choose to install in-house capabilities for the development of the drugs for better control and to save cost but CDMOs remain an integral part of manufacturing the drugs with more amount of experience and expertise as the development of advanced therapy products is a complicated process. CDMOs eventually, however, do charge an exorbitant price to cover their expenses but as a result, they provide high-quality manufacturing services, transparency, and expertise.
Product Insights
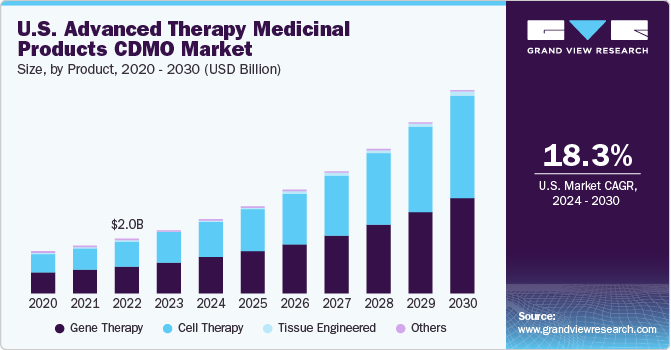
Genetherapyaccounted for the largest revenue share of over 50.0% in 2020. The rapid growth of the segment is attributed to the advancements in therapy as the treatment can alter and improve the genetics or specifically modify the targeted therapeutic treatment. Another factor promoting growth is the rising awareness leading to patients demanding this therapy, even in clinical stages.
In the last few years, gene therapy has observed lucrative growth due to its efficiency in invading cells and initiating genetic materials. The most common method used in gene therapy is recombinant DNA technology. Using genes as a treatment can address the cause of the disease at a cellular level and the result can be witnessed in just one treatment, which can act as a breakthrough in a patient’s life.
Indication Insights
Oncology accounted for the largest revenue share of over 45.0% in 2020. The growth of the segment is attributed to the increasing prevalence of cancer and chronic diseases owing to the rising geriatric population. Oncology is a branch of medicine, which diagnoses and treats cancer. The application of ATMPs was first initiated to find a breakthrough in cancer treatment, and hence over the years, this segment has the highest-level expertise as well as activity.
According to the WHO, cancer is the second-largest leading cause of death globally and nearly 10 million people died due to cancer in 2020. Most of the cancer cases have been found in undeveloped nations and it mainly affects the low- or middle-income countries due to the lack of a proper medical system. Similarly, the rate of cancer is low and the awareness regarding the diagnosis and treatment is high in countries where the health system is developed.
Phase Insights
Phase III accounted for the largest revenue share of over 50.0% in 2020. The growth of the segment is owing to the fact that phase III trials involve a large number of patients, and this phase is also the most extensive study period as it includes a comparison of the efficiency and safety of the new drug. An average Phase III trial tends to last for about one to four years.
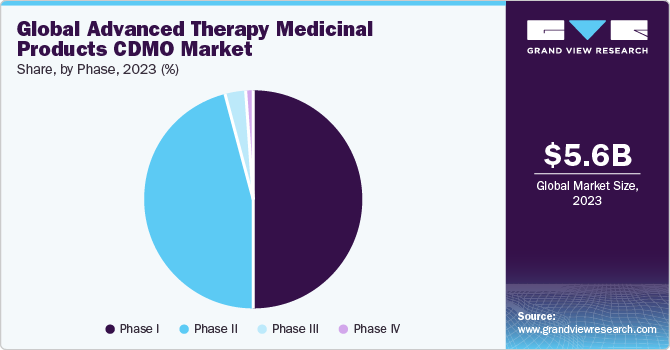
Phase I held the second-largest revenue share in 2020 owing to the growing R&D activities. With the advent of the Covid-19 pandemic and the use of ATMPs in the treatment of infectious diseases, the segment is expected to witness lucrative growth over the next 2-3 years. Phase I is conducted to check the level of safety of the drug in different dose levels for a small number of patients. This phase is mainly to check the highest dose a patient can take without any side effects. Around 70% of the phase, I drug moves to the next phase.
Regional Insights
North America held the largest revenue share of over 45.0% in 2020 owing to the growing awareness regarding advanced therapy and rising outsourcing activities. America has always been a pioneer in R&D activities for advanced treatment and is expected to maintain its lead over the forecast period.
The region has a favorable regulatory environment as the FDA has approved a few gene therapy products for sale in the U.S. such as ALLOCORD and ABECMA. According to the reports by American Biopharmaceutical Companies, about 362 cell and gene therapies are in the clinical pipeline of the U.S. as of 2020, out of which 132 are potentially for the treatment of rare diseases. Currently, the U.S. FDA has approved nine-cell and gene therapy products for advanced treatment.
Key Companies & Market Share Insights
As the demand for advanced therapy medicinal products increases, the number of companies willing to invest in such products is also expected to rise, leading to a high level of competition. The major market players mainly focus on service quality and expertise in carrying out the development and manufacturing of products to capture this growing market. For instance, in November 2020, Catalent has successfully acquired Bone Therapeutics’ cell therapy manufacturing subsidiary. The acquisition will expand the cell therapy capabilities of Catalent allowing them to take up more projects within this space. Some prominent players in the global advanced therapy medicinal products CDMO market include:
Celonic
Bio Elpida
CGT Catapult
Rentschler Biopharma SE
AGC Biologics
Catalent
Lonza
WuXi Advanced Therapies
BlueReg
Minaris Regenerative Medicine
Patheon
Advanced Therapy Medicinal Products CDMO Market ReportScope
Report Attribute |
Details |
Market size value in 2021 |
USD 5.84 billion |
Revenue forecast in 2028 |
USD 12.94 billion |
Growth Rate |
CAGR of 12.0% from 2021 to 2028 |
Base year for estimation |
2020 |
Historical data |
2016 - 2019 |
Forecast period |
2021 - 2028 |
Quantitative units |
Revenue in USD billion and CAGR from 2021 to 2028 |
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
Segments covered |
Product, phase, indication, region |
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
Country scope |
U.S.; Canada; Germany; U.K.; France; Italy; Spain; Japan; China; India; Australia; South Korea; Brazil; Mexico; Argentina; Colombia; Chile; South Africa; Saudi Arabia; UAE; Israel; Egypt |
Key companies profiled |
Celonic; Bio Elpida; CGT Catapult; Rentschler Biopharma SE; AGC Biologics; Catalent; Lonza; WuXi Advanced Therapies; BlueReg; Minaris Regenerative Medicine; Patheon |
Customization scope |
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional, and segment scope. |
革命制度党cing and purchase options |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
Segments Covered in the Report
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends and opportunities in each of the sub-segments from 2016 to 2028. For the purpose of this study, Grand View Research has segmented the global advanced therapy medicinal products CDMO market report on the basis of product, phase, indication, and region:
Product Outlook (Revenue, USD Billion, 2016 - 2028)
Gene Therapy
Cell Therapy
Tissue Engineered
Others (Combined ATMPs, for example biodegradable matric or scaffold)
Phase Outlook (Revenue, USD Billion, 2016 - 2028)
Phase I
Phase II
Phase III
Phase IV
Indication Outlook (Revenue, USD Billion, 2016 - 2028)
Oncology
Cardiology
Central Nervous System
Musculoskeletal
Infectious Disease
Dermatology
Endocrine, Metabolic, Genetic
Immunology & Inflammation
Ophthalmology
Haematology
Gastroenterology
Others
Regional Outlook (Revenue, USD Billion, 2016 - 2028)
North America
U.S.
Canada
Europe
U.K.
Germany
France
Italy
Spain
Asia Pacific
China
Japan
India
Australia
South Korea
Latin America
Brazil
Mexico
Argentina
Colombia
Chile
Middle East & Africa
South Africa
Saudi Arabia
UAE
Israel
Egypt
Frequently Asked Questions About This Report
b.The global advanced therapy medicinal products CDMO market size was estimated at USD 5.29 billion in 2020 and is expected to reach USD 5.84 billion in 2021.
b.The global advanced therapy medicinal products CDMO market is expected to grow at a compound annual growth rate of 12% from 2021 to 2028 to reach USD 12.94 billion by 2028.
b.Gene therapy dominated the advanced therapy medicinal products CDMO market with a share of 53.5% in 2020. This is attributable to the fact of advancement in therapy as the treatment can alter and improve the genetics or specifically modify the targeted therapeutical treatment.
b.Some key players operating in the advanced therapy medicinal products CDMO market include Celonic, Bio Elpida, CGT Catapult, Rentschler Biopharma SE, AGC Biologics, Catalent, Lonza.
b.Key factors that are driving the advanced therapy medicinal products CDMO market growth include increasing prevalence of rare & life-threatening diseases such as metabolic and optical disease, and rising investment in R&D of advanced therapy medicinal products.





